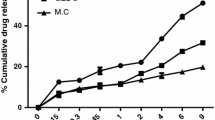
Abstract
Essential oils (EO) are aromatic compounds from the plant secondary metabolism. Melaleuca alternifolia EO is well known for its medicinal properties and promising use as an antimicrobial agent. Pythiosis is a difficult-to-treat and emerging disease caused by the oomycete Pythium insidiosum. This study evaluated a nanoemulsion formulation of M. alternifolia (NEMA) in topical and intralesional application to treat experimental pythiosis. Dermal toxicity tests were performed on M. alternifolia EO in Wistar rats. Pythiosis was reproduced in rabbits (n = 9) that were divided into groups: group 1 (control), cutaneous lesions with daily topical application of a non-ionizable gel-based formulation and intralesional application of sterile distilled water every 48 h; group 2 (topical formulation), lesions treated daily with topical application of a non-ionizable gel-based formulation containing 5 mg/ml of NEMA; and group 3 (intralesional formulation), lesions treated with NEMA at 5 mg/ml in aqueous solution applied intralesionally/48 h. The animals were treated for 45 days, and the subcutaneous lesion areas were measured every 5 days. M. alternifolia EO showed no dermal toxicity. The lesion areas treated with intralesional NEMA reduced at the end of treatment, differing from groups 1 and 2 (P < 0.05). In the topically treated group, the lesion areas did not differ from the control group, although the number of hyphae significantly reduced (P < 0.05). Under the experimental conditions of this study, the NEMA formulations presented a favorable safety profile. However, further studies are required to evaluate if this safety applies to higher concentrations of NEMA and to validate its use in clinical pythiosis.


Similar content being viewed by others
References
Bakkali F (2008) Biological effects of essential oils - a review. Food Chem Toxicol 46:446–475. https://doi.org/10.1016/j.fct.2007.09.106
Amann S, Neef K, Kohl S (2019) Antimicrobial resistance (AMR). Eur J Hosp Pharm 26:175–177. https://doi.org/10.1136/ejhpharm-2018-001820
Kozics K, Bučková M, Puškárová A et al (2019) The effect of ten essential oils on several cutaneous Drug-resistant microorganisms and their cyto/genotoxic and antioxidant properties. Molecules 24:4570. https://doi.org/10.3390/molecules24244570
Carmo PHF, Costa MC, Franco PHC et al (2020) Essential oils of Taxandria fragrans and Melaleuca alternifolia have effective antidermatophytic activities in vitro and in vivo that are antagonised by ketoconazole and potentiated in gold nanospheres. Nat Prod Res 2:1–4. https://doi.org/10.1080/14786419.2019.1709186
Casarin M, Pazinatto J, Oliveira LM et al (2019) Anti-biofilm and anti-inflammatory effect of a herbal nanoparticle mouthwash: a randomized crossover trial. Braz Oral Res 33:e062. https://doi.org/10.1590/1807-3107bor-2019.vol33.0062
Ireland DDJ, Greay SJ, Hooper CM et al (2012) Topically applied Melaleuca alternifolia (tea tree) oil causes direct anti-cancer cytotoxicity in subcutaneous tumour bearing mice. J Dermatol Sci 67:120–129. https://doi.org/10.1016/j.jdermsci.2012.05.005
Souza CF, Lima T, Baldissera MD et al (2018) Nanoencapsulated Melaleuca alternifolia essential oil exerts anesthetic effects in the brachyuran crab using Neohelice granulata. Academ Bras Ciências 90:2855–2864. https://doi.org/10.1590/0001-3765201820170930
Tullio V, Roana J, Scala SD et al (2019) Enhanced killing of Candida krusei by polymorphonuclear leucocytes in the presence of subinhibitory concentrations of Melaleuca alternifolia and "Mentha of Pancalieri" essential oils. Molecules 24:3824. https://doi.org/10.3390/molecules24213824
Teng L, Fan X, Nelson DR et al (2019) Diversity and evolution of cytochromes P450 in stramenopiles. Planta 249:647–661. https://doi.org/10.1007/s00425-018-3028-1
Gaastra W, Lipman LJ, De Cock AW et al (2010) Pythium insidiosum: an overview. Vet Microbiol 146:1–16. https://doi.org/10.1016/j.vetmic.2010.07.019
Souto EPF, Maia LA, Neto EGM et al (2021) Pythiosis in Equidae in Northeastern Brazil: 1985-2020. J Equine Vet Sci 105:103726. https://doi.org/10.1016/j.jevs.2021.103726
Hilton RE, Tepedino K, Glenn CJ et al (2016) Swamp cancer: a case of human pythiosis and review of the literature. Br J Dermatol 175:394–397. https://doi.org/10.1111/bjd.14520
Pereira DIB, Santurio JM, Alves SH et al (2007) Caspofungin in vitro and in vivo activity against Brazilian Pythium insidiosum strains isolated from animals. J Antimicrob Chemother 60:1168–1171. https://doi.org/10.1093/jac/dkm332
Zanette RA, Jesus FP, Pilotto MB et al (2015) Micafungin alone and in combination therapy with deferasirox against Pythium insidiosum. J Mycol Med 25:91–94. https://doi.org/10.1016/j.mycmed.2014.09.002
Itaqui SR, Verdi CM, Tondolo JS et al (2016) In vitro synergism between azithromycin or terbinafine and topical antimicrobial agents against Pythium insidiosum. Antimicrob Agents Chemother 60:5023–5025. https://doi.org/10.1128/AAC.00154-16
Jesus FP, Loreto ES, Ferreiro L et al (2016) In vitro and in vivo antimicrobial activities of minocycline in combination with azithromycin, clarithromycin, or tigecycline against Pythium insidiosum. Antimicrob Agents Chemother 60:87–91. https://doi.org/10.1128/AAC.01480-15
Bagga B, Sharma S, Guda SJM et al (2018) Leap forward in the treatment of Pythium insidiosum keratitis. British J Ophthalmol 102:1629–1633. https://doi.org/10.1136/bjophthalmol-2017-311360
Chatterjee S, Agrawal D (2018) Azitromycin in the management of Pythium insidiosum keratitis. Cornea 37:e8–e9. https://doi.org/10.1097/ICO.0000000000001419
Loreto ES, Tondolo JSM, Oliveira DC et al (2018) In vitro activities of miltefosine and antibacterial agents from the macrolide, oxazolidinone and pleuromutilin classes against Pythium insidiosum and Pythium aphanidermatum. Antimicrob Agents Chemother 62:1678–1717. https://doi.org/10.1128/AAC.01678-17
Loreto ES, Tondolo JSM, Jesus FPK et al (2019) Efficacy of miltefosine therapy against subcutaneous experimental pythiosis in rabbits. J Mycol Med 30:1009–1019. https://doi.org/10.1016/j.mycmed.2019.100919
Fonseca AO, Pereira DI, Botton SA et al (2015) Treatment of experimental pythiosis with essential oils of Origanum vulgare and Mentha piperita singly, in association and in combination with immunotherapy. Vet Microbiol 178:265–269. https://doi.org/10.1016/j.vetmic.2015.05.023
Araujo MJAM, Bosco SMG, Sforcin JM (2016) Pythium insidiosum: inhibitory effects of propolis and geopropolis on hyfal growth. Braz J Microbiol 47:863–869. https://doi.org/10.1016/j.bjm.2016.06.008
Valente JSS, Fonseca AO, Brasil CL et al (2016a) In vitro activity of Melaleuca alternifolia (tea tree) in its free oil and nanoemulsion formulations against Pythium insidiosum. Mycopathologia 181:865–869. https://doi.org/10.1007/s11046-016-0051-2
Valente JSS, Fonseca AO, Denardi LB et al (2016b) In vitro susceptibility of Pythium insidiosum to Melaleuca alternifolia, Mentha piperita and Origanum vulgare essential oils combinations. Mycopathologia 181:5–6. https://doi.org/10.1007/s11046-016-0019-2
Trolezi R, Azanha JM, Paschoal NR et al (2017) Stryphnodendron adstringens and purified tannin on Pythium insidiosum:in vitro and in vivo studies. Ann Clin Microbiol Antimicrob 1:1–7. https://doi.org/10.1186/s12941-017-0183-3
Valente JSS, Brasil CL, Braga CQ et al (2020) Biogenic silver nanoparticles in the treatment of experimental Pythiosis. Med Mycol 58:913–918. https://doi.org/10.1093/mmy/myz141
Bouchemal K, Briançon S, Perrier E et al (2004) Nano-emulsion formulation using spontaneous emulsification: solvent, oil and surfactant optimization. Int J Pharm 280:241–251. https://doi.org/10.1016/j.ijpharm.2004.05.016
Flores FC, Ribeiro RF, Ourique AF et al (2011) Nanostructured systems containing an essential oil: protection against volatilization. Química Nova 34:968–972. https://doi.org/10.1590/S0100-40422011000600010
OECD (Organization for Economic Cooperation and Development): Guideline for the testing of chemicals, 410: “repeated dose dermal toxicity: 21/28-day study”, Adopted 12 May 1981
Statistical Analysis System (SAS) (2019) SAS/STAT user guide, Version 9.4. Cary, NC: SAS Institute
Spinelli MO, Motta MC, Cruz RJ et al (2012) Estudo dos analitos bioquímicos no plasma de coelhos (Nova Zelândia) mantidos no biotério da Faculdade de Medicina da Universidade de São Paulo. Soc Bras Cienc Anim Lab (SBCAL) 1:163–168
Ameri M, Schnaars HA, Sibley JR et al (2011) Determination of plasma fibrinogen concentrations in beagle dogs, Cynomolgus monkeys, New Zealand white rabbits, and Sprague-Dawley rats by using Clauss and prothrombin-time-derived assays. J Am Assoc Labo Anim Sci 50:864–867
Emanuelli M, Lopes ST, Maciel R et al (2008) Concentração sérica de fosfatase alcalina, gama-glutamil transferase, uréia e creatinina em coelhos (Oryctolagus cuniculus). Ciênc Anim Bras 9:251–255
Pisseri F, Bertolib A, Nardonic S et al (2009) Antifungal activity of tea tree oil from Melaleuca alternifolia against Trichophyton equinum: An in vivo assay. Phytomedicine 16:1056–1058. https://doi.org/10.1016/j.phymed.2009.03.013
Swamy MK, Akhtar MS, Sinniah UR (2016) Antimicrobial properties of plant essential oils against human pathogens and their mode of action: an updated review. Evid Based Complement Alternat Med 1:1–21. https://doi.org/10.1155/2016/3012462
Kong Q, Zhang L, An P et al (2019) Antifungal mechanisms of a-terpineol and terpene-4-alcohol as the critical components of Melaleuca alternifolia oil in the inhibition of rot disease caused by Aspergillus ochraceus in postharvest grapes. J Appl Microbiol 126:1161–1174. https://doi.org/10.1111/jam.14193
Bezdjian A, Mujica-mota MA, Azzi M et al (2014) Assessment of ototoxicity of tea tree oil in a chinchilla animal model. Int J Pediatr Otorhinolaryngol 78:2136–2139. https://doi.org/10.1016/j.ijporl.2014.09.023
Funding
This research was supported by Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES - Finance Code 001) and National Council for Scientific and Technological Development – CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for student and researcher scholarships.
Author information
Authors and Affiliations
Contributions
Silveira JS, Pereira DIB, and Botton SA conceived the idea, performed the experiment, collected and interpreted data, and wrote the manuscript. Silveira JS, Brasil CL, Braga CQ, Moreira AS, Albano AP, and Zambrano CG performed the animal experiment. Zamboni R and Sallis ES performed necropsy and histopathological analysis. Franz HC performed hematological and biochemical analysis. Silva CB and Araujo LC produced Melaleuca alternifolia nanoemulsion. Pötter L. performed the statistical analysis, interpreted data, and wrote the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All applicable institutional guidelines for the care and use of animals were followed (Ethics Committee of Federal University of Pelotas – CEEA - n° 23110.002723/2016-77).
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Luiz Henrique Rosa
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 26 kb)
Rights and permissions
About this article
Cite this article
de Souza Silveira, J., Brasil, C.L., Braga, C.Q. et al. Melaleuca alternifolia formulations in the treatment of experimental pythiosis. Braz J Microbiol 53, 1011–1017 (2022). https://doi.org/10.1007/s42770-022-00720-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-022-00720-6