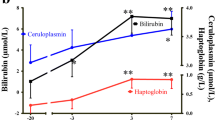
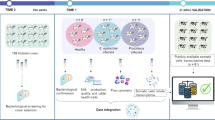
Abstract
Bovine mastitis, an inflammatory disease of the mammary gland, is classified as subclinical or clinical. Circulating neutrophils are recruited to the udder to combat infection. We compared the transcriptomic profiles in circulating leukocytes between healthy cows and those with naturally occurring subclinical or clinical mastitis. Holstein Friesian dairy cows from six farms in EU countries were recruited. Based on milk somatic cell count and clinical records, cows were classified as healthy (n = 147), subclinically (n = 45) or clinically mastitic (n = 22). Circulating leukocyte RNA was sequenced with Illumina NextSeq single end reads (30 M). Differentially expressed genes (DEGs) between the groups were identified using CLC Genomics Workbench V21, followed by GO enrichment analysis. Both subclinical and clinical mastitis caused significant changes in the leukocyte transcriptome, with more intensive changes attributed to clinical mastitis. We detected 769 DEGs between clinical and healthy groups, 258 DEGs between subclinical and healthy groups and 193 DEGs between clinical and subclinical groups. Most DEGs were associated with cell killing and immune processes. Many upregulated DEGs in clinical mastitis encoded antimicrobial peptides (AZU1, BCL3, CAMP, CATHL1, CATHL2, CATHL4,CATHL5, CATHL6, CCL1, CXCL2, CXCL13, DEFB1, DEFB10, DEFB4A, DEFB7, LCN2, PGLYRP1, PRTN3, PTX3, S100A8, S100A9, S100A12, SLC11A1, TF and LTF) which were not upregulated in subclinical mastitis. The use of transcriptomic profiles has identified a much greater up-regulation of genes encoding antimicrobial peptides in circulating leukocytes of cows with naturally occurring clinical compared with subclinical mastitis. These could play a key role in combatting disease organisms.

Similar content being viewed by others
Data availability
The sequencing data were deposited to the European Nucleotide Archive (ERP019874).
Abbreviations
- AMP:
-
Antimicrobial peptide
- BH:
-
Benjamini Hochberg
- DEG:
-
Differentially expressed gene
- DIM:
-
Days in milk
- GO:
-
Gene ontology
- LDH:
-
Lactate dehydrogenase
- NAGase:
-
N-acetyl-β-d-glucosaminidase
- PAMP:
-
Pathogen-associated molecular pattern
- PMNL:
-
Polymorphonuclear leukocytes
- SC:
-
Somatic cells
- SCC:
-
Somatic cell count
References
Oviedo-Boyso J, Valdez-Alarcon JJ, Cajero-Juarez M, Ochoa-Zarzosa A, Lopez-Meza JE, Bravo-Patino A, Baizabal-Aguirre VM (2007) Innate immune response of bovine mammary gland to pathogenic bacteria responsible for mastitis. J Infect 54(4):399–409. https://doi.org/10.1016/j.jinf.2006.06.010
Ruegg PL (2017) A 100-year review: mastitis detection, management, and prevention. J Dairy Sci 100(12):10381–10397. https://doi.org/10.3168/jds.2017-13023
Lawless N, Reinhardt TA, Bryan K, Baker M, Pesch B, Zimmerman D, Zuelke K, Sonstegard T, O’Farrelly C, Lippolis JD, Lynn DJ (2014) MicroRNA regulation of bovine monocyte inflammatory and metabolic networks in an in vivo infection model. G3 (Bethesda) 4(6):957–971. https://doi.org/10.1534/g3.113.009936
Wells SJ, Ott SL, Seitzinger AH (1998) Key health issues for dairy cattle-new and old. J Dairy Sci 81(11):3029–3035. https://doi.org/10.3168/jds.s0022-0302(98)75867-9
Bradley A (2002) Bovine mastitis: an evolving disease. Vet J 164(2):116–128. https://doi.org/10.1053/tvjl.2002.0724
Reinoso EB, Lasagno MC, Dieser SA, Odierno LM (2011) Distribution of virulence-associated genes in Streptococcus uberis isolated from bovine mastitis. FEMS Microbiol Lett 318(2):183–188. https://doi.org/10.1111/j.1574-6968.2011.02258.x
Ward PN, Holden MT, Leigh JA, Lennard N, Bignell A, Barron A, Clark L, Quail MA, Woodward J, Barrell BG, Egan SA, Field TR, Maskell D, Kehoe M, Dowson CG, Chanter N, Whatmore AM, Bentley SD, Parkhill J (2009) Evidence for niche adaptation in the genome of the bovine pathogen Streptococcus uberis. BMC Genomics 10:54. https://doi.org/10.1186/1471-2164-10-54
Pyorala S (2002) New strategies to prevent mastitis. Reprod Domest Anim 37(4):211–216. https://doi.org/10.1046/j.1439-0531.2002.00378.x
Leitner G, Yadlin B, Glickman A, Chaffer M, Saran A (2000) Systemic and local immune response of cows to intramammary infection with Staphylococcus aureus. Res Vet Sci 69(2):181–184. https://doi.org/10.1053/rvsc.2000.0409
Thompson-Crispi K, Atalla H, Miglior F, Mallard BA (2014) Bovine mastitis: frontiers in immunogenetics. Front Immunol 5:493. https://doi.org/10.3389/fimmu.2014.00493
Schukken YH, Wilson DJ, Welcome F, Garrison-Tikofsky L, Gonzalez RN (2003) Monitoring udder health and milk quality using somatic cell counts. Vet Res 34(5):579–596. https://doi.org/10.1051/vetres:2003028
Heimes A, Brodhagen J, Weikard R, Seyfert HM, Becker D, Meyerholz MM, Petzl W, Zerbe H, Hoedemaker M, Rohmeier L, Schuberth HJ, Schmicke M, Engelmann S, Kuhn C (2020) Hepatic transcriptome analysis identifies divergent pathogen-specific targeting-strategies to modulate the innate immune system in response to intramammary infection. Front Immunol 11:715. https://doi.org/10.3389/fimmu.2020.00715
Jiang L, Sorensen P, Rontved C, Vels L, Ingvartsen KL (2008) Gene expression profiling of liver from dairy cows treated intra-mammary with lipopolysaccharide. BMC Genomics 9:443. https://doi.org/10.1186/1471-2164-9-443
Moyes KM, Sorensen P, Bionaz M (2016) The Impact of intramammary Escherichia coli challenge on liver and mammary transcriptome and cross-talk in dairy cows during early lactation using RNAseq. PLoS One 11(6):e0157480. https://doi.org/10.1371/journal.pone.0157480
Rinaldi M, Li RW, Capuco AV (2010) Mastitis associated transcriptomic disruptions in cattle. Vet Immunol Immunopathol 138(4):267–279. https://doi.org/10.1016/j.vetimm.2010.10.005
Chen X, Cheng Z, Zhang S, Werling D, Wathes DC (2015) Combining genome wide association studies and differential gene expression data analyses identifies candidate genes affecting mastitis caused by two different pathogens in the dairy cow. Open J Anim Sci 05(04):358–393. https://doi.org/10.4236/ojas.2015.54040
Bruckmaier RM, Wellnitz O (2017) TRIENNIAL LACTATION SYMPOSIUM/BOLFA: pathogen-specific immune response and changes in the blood–milk barrier of the bovine mammary gland. J Anim Sci 95(12):5720–5728. https://doi.org/10.2527/jas2017.1845
Sheldon IM, Lewis GS, LeBlanc S, Gilbert RO (2006) Defining postpartum uterine disease in cattle. Theriogenology 65(8):1516–1530. https://doi.org/10.1016/j.theriogenology.2005.08.021
Vangroenweghe F, Lamote I, Burvenich C (2005) Physiology of the periparturient period and its relation to severity of clinical mastitis. Domest Anim Endocrinol 29(2):283–293. https://doi.org/10.1016/j.domaniend.2005.02.016
Mallard BA, Dekkers JC, Ireland MJ, Leslie KE, Sharif S, Vankampen CL, Wagter L, Wilkie BN (1998) Alteration in immune responsiveness during the peripartum period and its ramification on dairy cow and calf health. J Dairy Sci 81(2):585–595. https://doi.org/10.3168/jds.s0022-0302(98)75612-7
Wathes DC, Cheng Z, Chowdhury W, Fenwick MA, Fitzpatrick R, Morris DG, Patton J, Murphy JJ (2009) Negative energy balance alters global gene expression and immune responses in the uterus of postpartum dairy cows. Physiol Genomics 39(1):1–13. https://doi.org/10.1152/physiolgenomics.00064.2009
Ingvartsen KL, Moyes K (2013) Nutrition, immune function and health of dairy cattle. Animal 7(Suppl 1):112–122. https://doi.org/10.1017/S175173111200170X
Ster C, Loiselle MC, Lacasse P (2012) Effect of postcalving serum nonesterified fatty acids concentration on the functionality of bovine immune cells. J Dairy Sci 95(2):708–717. https://doi.org/10.3168/jds.2011-4695
Kehrli ME Jr, Shuster DE (1994) Factors affecting milk somatic cells and their role in health of the bovine mammary gland. J Dairy Sci 77(2):619–627. https://doi.org/10.3168/jds.S0022-0302(94)76992-7
Lacetera N, Scalia D, Bernabucci U, Ronchi B, Pirazzi D, Nardone A (2005) Lymphocyte functions in overconditioned cows around parturition. J Dairy Sci 88(6):2010–2016. https://doi.org/10.3168/jds.S0022-0302(05)72877-0
Nonnecke BJ, Kimura K, Goff JP, Kehrli ME Jr (2003) Effects of the mammary gland on functional capacities of blood mononuclear leukocyte populations from periparturient cows. J Dairy Sci 86(7):2359–2368. https://doi.org/10.3168/jds.S0022-0302(03)73829-6
Mitterhuemer S, Petzl W, Krebs S, Mehne D, Klanner A, Wolf E, Zerbe H, Blum H (2010) Escherichia coli infection induces distinct local and systemic transcriptome responses in the mammary gland. BMC Genomics 11:138. https://doi.org/10.1186/1471-2164-11-138
Petzl W, Zerbe H, Gunther J, Seyfert HM, Hussen J, Schuberth HJ (2018) Pathogen-specific responses in the bovine udder. Models and immunoprophylactic concepts. Res Vet Sci 116:55–61. https://doi.org/10.1016/j.rvsc.2017.12.012
De Koster J, Salavati M, Grelet C, Crowe MA, Matthews E, O’Flaherty R, Opsomer G, Foldager L, GplusE HM (2019) Prediction of metabolic clusters in early-lactation dairy cows using models based on milk biomarkers. J Dairy Sci 102(3):2631–2644. https://doi.org/10.3168/jds.2018-15533
Krogh MA, Hostens M, Salavati M, Grelet C, Sorensen MT, Wathes DC, Ferris CP, Marchitelli C, Signorelli F, Napolitano F, Becker F, Larsen T, Matthews E, Carter F, Vanlierde A, Opsomer G, Gengler N, Dehareng F, Crowe MA, Ingvartsen KL, Foldager L (2020) Between- and within-herd variation in blood and milk biomarkers in Holstein cows in early lactation. Animal 14(5):1067–1075. https://doi.org/10.1017/S1751731119002659
Larsen T, Rontved CM, Ingvartsen KL, Vels L, Bjerring M (2010) Enzyme activity and acute phase proteins in milk utilized as indicators of acute clinical E. coli LPS-induced mastitis. Animal 4(10):1672–1679. https://doi.org/10.1017/S1751731110000947
Green MJ, Bradley AJ, Medley GF, Browne WJ (2007) Cow, farm, and management factors during the dry period that determine the rate of clinical mastitis after calving. J Dairy Sci 90(8):3764–3776. https://doi.org/10.3168/jds.2007-0107
Steeneveld W, Hogeveen H, Barkema HW, van den Broek J, Huirne RB (2008) The influence of cow factors on the incidence of clinical mastitis in dairy cows. J Dairy Sci 91(4):1391–1402. https://doi.org/10.3168/jds.2007-0705
Luoreng ZM, Wang XP, Mei CG, Zan LS (2018) Expression profiling of peripheral blood miRNA using RNAseq technology in dairy cows with Escherichia coli-induced mastitis. Sci Rep 8(1):12693. https://doi.org/10.1038/s41598-018-30518-2
Wang D, Liu L, Augustino SMA, Duan T, Hall TJ, MacHugh DE, Dou J, Zhang Y, Wang Y, Yu Y (2020) Identification of novel molecular markers of mastitis caused by Staphylococcus aureus using gene expression profiling in two consecutive generations of Chinese Holstein dairy cattle. J Anim Sci Biotechnol 11:98. https://doi.org/10.1186/s40104-020-00494-7
Yalcin C, Stott AW, Logue DN, Gunn J (1999) The economic impact of mastitis-control procedures used in Scottish dairy herds with high bulk-tank somatic-cell counts. Prev Vet Med 41(2–3):135–149. https://doi.org/10.1016/s0167-5877(99)00052-5
Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, Wilkinson KA, Banchereau R, Skinner J, Wilkinson RJ, Quinn C, Blankenship D, Dhawan R, Cush JJ, Mejias A, Ramilo O, Kon OM, Pascual V, Banchereau J, Chaussabel D, O’Garra A (2010) An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466(7309):973–977. https://doi.org/10.1038/nature09247
Blankley S, Berry MP, Graham CM, Bloom CI, Lipman M, O’Garra A (2014) The application of transcriptional blood signatures to enhance our understanding of the host response to infection: the example of tuberculosis. Philos Trans R Soc Lond B Biol Sci 369(1645):20130427. https://doi.org/10.1098/rstb.2013.0427
Schukken YH, Gunther J, Fitzpatrick J, Fontaine MC, Goetze L, Holst O, Leigh J, Petzl W, Schuberth HJ, Sipka A, Smith DG, Quesnell R, Watts J, Yancey R, Zerbe H, Gurjar A, Zadoks RN, Seyfert HM, members of the Pfizer mastitis research c (2011) Host-response patterns of intramammary infections in dairy cows. Vet Immunol Immunopathol 144(3–4):270–289. https://doi.org/10.1016/j.vetimm.2011.08.022
Bannerman DD (2009) Pathogen-dependent induction of cytokines and other soluble inflammatory mediators during intramammary infection of dairy cows. J Anim Sci 87(13 Suppl):10–25. https://doi.org/10.2527/jas.2008-1187
Pasupuleti M, Schmidtchen A, Malmsten M (2012) Antimicrobial peptides: key components of the innate immune system. Crit Rev Biotechnol 32(2):143–171. https://doi.org/10.3109/07388551.2011.594423
Risso A (2000) Leukocyte antimicrobial peptides: multifunctional effector molecules of innate immunity. J Leukoc Biol 68(6):785–792
Afacan NJ, Yeung AT, Pena OM, Hancock RE (2012) Therapeutic potential of host defense peptides in antibiotic-resistant infections. Curr Pharm Des 18(6):807–819. https://doi.org/10.2174/138161212799277617
Auvynet C, Rosenstein Y (2009) Multifunctional host defense peptides: antimicrobial peptides, the small yet big players in innate and adaptive immunity. FEBS J 276(22):6497–6508. https://doi.org/10.1111/j.1742-4658.2009.07360.x
Hilchie AL, Wuerth K, Hancock RE (2013) Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol 9(12):761–768. https://doi.org/10.1038/nchembio.1393
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3(3):238–250. https://doi.org/10.1038/nrmicro1098
Kosciuczuk EM, Lisowski P, Jarczak J, Strzalkowska N, Jozwik A, Horbanczuk J, Krzyzewski J, Zwierzchowski L, Bagnicka E (2012) Cathelicidins: family of antimicrobial peptides. A review. Mol Biol Rep 39(12):10957–10970. https://doi.org/10.1007/s11033-012-1997-x
Andreu D, Rivas L (1998) Animal antimicrobial peptides: an overview. Biopolymers 47(6):415–433. https://doi.org/10.1002/(SICI)1097-0282(1998)47:6%3c415::AID-BIP2%3e3.0.CO;2-D
Gurao A, Kashyap SK, Singh R (2017) beta-defensins: an innate defense for bovine mastitis. Vet World 10(8):990–998. https://doi.org/10.14202/vetworld.2017.990-998
Stanton TB (2013) A call for antibiotic alternatives research. Trends Microbiol 21(3):111–113. https://doi.org/10.1016/j.tim.2012.11.002
Young-Speirs M, Drouin D, Cavalcante PA, Barkema HW, Cobo ER (2018) Host defense cathelicidins in cattle: types, production, bioactive functions and potential therapeutic and diagnostic applications. Int J Antimicrob Agents 51(6):813–821. https://doi.org/10.1016/j.ijantimicag.2018.02.006
Loftus RM, Finlay DK (2016) Immunometabolism: cellular metabolism turns immune regulator. J Biol Chem 291(1):1–10. https://doi.org/10.1074/jbc.R115.693903
Habel J, Sundrum A (2020) Mismatch of glucose allocation between different life functions in the transition period of dairy cows. Animals (Basel) 10(6):1028–1049. https://doi.org/10.3390/ani10061028
LeBlanc SJ (2012) Interactions of metabolism, inflammation, and reproductive tract health in the postpartum period in dairy cattle. Reprod Domest Anim 47(Suppl 5):18–30. https://doi.org/10.1111/j.1439-0531.2012.02109.x
Moyes KM, Larsen T, Ingvartsen KL (2013) Generation of an index for physiological imbalance and its use as a predictor of primary disease in dairy cows during early lactation. J Dairy Sci 96(4):2161–2170. https://doi.org/10.3168/jds.2012-5646
Bradley AJ, Leach KA, Breen JE, Green LE, Green MJ (2007) Survey of the incidence and aetiology of mastitis on dairy farms in England and Wales. Vet Rec 160(8):253–257. https://doi.org/10.1136/vr.160.8.253
Acknowledgements
GplusE Consortium: Mark Crowe, Niamh McLoughlin, Alan Fahey, Elizabeth Matthews, Andreia Santoro, Colin Byrne, Pauline Rudd, Roisin O’Flaherty, Sinead Hallinan, Claire Wathes, Zhangrui Cheng, Ali Fouladi, Geoff Pollott, Dirk Werling, Beatriz Sanz Bernardo, Mazdak Salavati, Laura Buggiotti, Alistair Wylie, Matt Bell, Mieke Vaneetvelde, Kristof Hermans, Geert Opsomer, Sander Moerman, Jenne De Koster, Hannes Bogaert, Jan Vandepitte, Leila Vandevelde, Bonny Vanranst, Johanna Hoglund, Susanne Dahl, Klaus Ingvartsen, Martin Sørensen, Leslie Foldager, Soren Ostergaard, Janne Rothmann, Mogens Krogh, Else Meyer, Charlotte Gaillard, Jehan Ettema, Tine Rousing, Federica Signorelli, Francesco Napolitano, Bianca Moioli, Alessandra Crisa, Luca Buttazzoni, Jennifer McClure, Daragh Matthews, Francis Kearney, Andrew Cromie, Matt McClure, Shujun Zhang, Xing Chen, Huanchun Chen, Junlong Zhao, Liguo Yang, Guohua Hua, Chen Tan, Guiqiang Wang, Michel Bonneau, Andrea Pompozzi, Armin Pearn, Arnold Evertson, Linda Kosten, Anders Fogh, Thomas Andersen, Matthew Lucy, Chris Elsik, Gavin Conant, Jerry Taylor, Nicolas Gengler, Michel Georges, Frederic Colinet, Marilou Ramos Pamplona, Hedi Hammami, Catherine Bastin, Haruko Takeda, Aurelie Laine, Anne-Sophie Van Laere, Martin Schulze, Cinzia Marchitelli and Sergio Palma-Vera.
Funding
This project received funding from the European Union’s Seventh Framework Programme (Brussels, Belgium) for research, technological development, and demonstration under Grant Agreement No. 613689. The views expressed in this publication are the sole responsibility of the authors and do not necessarily reflect the views of the European Commission.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures had local ethical approval and complied with the relevant national and EU legislation under European Union Directive 2010/63/EU.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The members of GplusE Consortium are listed in Acknowledgements section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cheng, Z., Buggiotti, L., Salavati, M. et al. Global transcriptomic profiles of circulating leucocytes in early lactation cows with clinical or subclinical mastitis. Mol Biol Rep 48, 4611–4623 (2021). https://doi.org/10.1007/s11033-021-06494-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06494-8