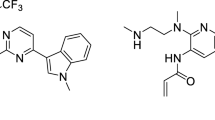
Abstract
SH-1028 is an irreversible third-generation EGFR tyrosine kinase inhibitor (EGFR-TKI) for the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC). Considering the possibility of combination therapy in patients with NSCLC, we investigated the drug-drug interaction (DDI) potential of SH-1028 both in vitro and in clinical trials. The in vitro studies were conducted to determine the potential of SH-1028 as a substrate, inducer, or inhibitor of cytochrome P450 (CYP) subtypes. A phase I drug-drug interaction study in healthy volunteers was performed to evaluate the impact of co-administering rifampicin (a strong CYP3A4 inducer) and itraconazole (a strong CYP3A4 inhibitor) on the pharmacokinetics of SH-1028. The in vitro experiments showed that SH-1028 was mainly metabolized by CYP3A4. The activities of CYP1A2, 2B6, 2C19, 2D6 and 3A4 enzymes were slightly inhibited in vitro with SH-1028. SH-1028 has no obvious induction effect on CYP1A2 and CYP2B6 activities, but has potential induction effect on CYP3A4 mRNA expression. However, SH-1028 may not induce or inhibit human CYPs significantly at the clinically expected dose (200 mg). The geometric mean ratios of pharmacokinetic parameters and their corresponding 90% confidence intervals for SH-1028 in combination and alone did not fall within the range of 80–125%. It is speculated that itraconazole and rifampicin affect the metabolism of SH-1028. In the clinical application of SH-1028, special attention should be paid to the interaction between SH-1028 and drugs or foods that affect the activity of CYP3A4. (Clinical trial registration number: CTR20210558)



Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Spiro SG (2005) One hundred years of lung cancer. Am J Respir Crit Care Med 172(5):523–529. https://doi.org/10.1164/rccm.200504-531OE
Nasim F, Sabath BF (2019) Lung Cancer. Med Clin North Am 103(3):463–473. https://doi.org/10.1016/j.mcna.2018.12.006
Zappa C (2016) Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res 5(3):288–300. https://doi.org/10.21037/tlcr.2016.06.07
Molina JR, Yang P, Cassivi SD, Schild SE (2008) Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 83(5):584–594. https://doi.org/10.4065/83.5.584
Herbst RS, Morgensztern D (2018) The biology and management of non-small cell lung cancer. Nature 553(7689):446–454. https://doi.org/10.1038/nature25183
Imyanitov EN, Iyevleva AG (2021) Molecular testing and targeted therapy for non-small cell lung cancer: current status and perspectives. Crit Rev Oncol Hematol 157:103194. https://doi.org/10.1016/j.critrevonc.2020.103194
Liao BC, Lin CC, Lee JH (2017) Optimal management of EGFR-mutant non-small cell lung cancer with disease progression on first-line tyrosine kinase inhibitor therapy. Lung Cancer 110:7–13. https://doi.org/10.1016/j.lungcan.2017.05.009
Lee DH (2017) Treatments for EGFR-mutant non-small cell lung cancer (NSCLC): the road to a success, paved with failures. Pharmacol Ther 174:1–21. https://doi.org/10.1016/j.pharmthera.2017.02.001
Tan CS, Cho BC (2016) Next-generation epidermal growth factor receptor tyrosine kinase inhibitors in epidermal growth factor receptor -mutant non-small cell lung cancer. Lung Cancer 93:59–68. https://doi.org/10.1016/j.lungcan.2016.01.003
Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, Orme JP, Finlay MR, Ward RA, Mellor MJ, Hughes G, Rahi A, Jacobs VN, Brewer R, Ichihara M, Sun E, Jin J, Ballard H, Al-Kadhimi P, Rowlinson K, Klinowska R, Richmond T, Cantarini GH, Kim M, Ranson DW, Pao MR, W (2014) AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 4(9):1046–1061. https://doi.org/10.1158/2159-8290.Cd-14-0337
Ellis PM, Coakley N, Feld R, Kuruvilla S (2015) Use of the epidermal growth factor receptor inhibitors gefitinib, erlotinib, afatinib, dacomitinib, and icotinib in the treatment of non-small-cell lung cancer: a systematic review. Curr Oncol 22(3):e183–215. https://doi.org/10.3747/co.22.2566
Lamb YN (2021) Osimertinib: a review in previously untreated, EGFR Mutation-Positive, Advanced NSCLC. Target Oncol 16(5):687–695. https://doi.org/10.1007/s11523-021-00839-w
Wu SG (2018) Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer 17(1):38. https://doi.org/10.1186/s12943-018-0777-1
Han L, Zhang X, Wang Z, Zhang X, Zhao L, Fu W, Liang X, Zhang Z (2021) SH-1028, an irreversible third-generation EGFR TKI, overcomes T790M-Mediated resistance in Non-Small Cell Lung Cancer. Front Pharmacol 12:665253. https://doi.org/10.3389/fphar.2021.665253
Lu C (2020) In vitro and in vivo methods to assess pharmacokinetic drug- drug interactions in drug discovery and development. Biopharm Drug Dispos 41(1–2):3–31. https://doi.org/10.1002/bdd.2212
Neuvonen PJ (1996) Itraconazole drastically increases plasma concentrations of lovastatin and lovastatin acid. Clin Pharmacol Ther 60(1):54–61. https://doi.org/10.1016/s0009-9236(96)90167-8
Swaisland HC, Ranson M, Smith RP, Leadbetter J, Laight A, McKillop D (2005) Pharmacokinetic drug interactions of gefitinib with rifampicin, itraconazole and metoprolol. Clin Pharmacokinet 44(10):1067–1081. https://doi.org/10.2165/00003088-200544100-00005
Vishwanathan K, Dickinson PA, So K, Thomas K, Chen YM, De Castro Carpeño J, Dingemans AC, Kim HR, Kim JH, Krebs MG, Chih-Hsin Yang J, Bui K, Weilert D (2018) The effect of itraconazole and rifampicin on the pharmacokinetics of osimertinib. Br J Clin Pharmacol 84(6):1156–1169. https://doi.org/10.1111/bcp.13534
Funding
This work was supported by the “512 Talent Fund” of Bengbu Medical College (BY51201313, China) and the Research and development project commissioned by Nanjing Sanhome Pharmaceutical Co., Ltd(2021043).
Author information
Authors and Affiliations
Contributions
YY-L, ZQ-W and H-Z contributed to the conception and design. XL-L and YY-L provided medical supervision. MH-Z and CX-H drafted the manuscript, and YY-X conducted the analysis and interpretation of the data. JX-D designed figures and tables, and reviewed the grammar. Y-W contributed to the management of drug and biological sample disposition. RF-S contributed to quality control throughout the study. BY-L contributed to the study organization and implementation. YZ-D and J-X participated in the sample collection. All authors were involved in revising the paper critically for intellectual content and the final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of Interest
We confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Ethics approval
This study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the institutional review boards of all participating sites.
Consent to participate
Written informed consent was obtained from all the participants included in the study.
Consent to publish
Permission to submit the manuscript for publication was obtained from all named authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Liu, Y., Zhu, M. et al. Drug-drug interaction potential of SH-1028, a third-generation EGFR-TKI: in vitro and clinical trials. Invest New Drugs 41, 453–462 (2023). https://doi.org/10.1007/s10637-023-01356-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-023-01356-5