Summary
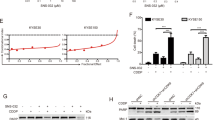
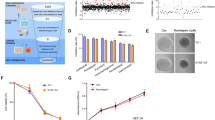
Esophageal squamous cell carcinoma (ESCC) is one of the most serious life-threatening malignancies. Although chemotherapeutic targets and agents for ESCC have made much progress recently, the efficacy is still unsatisfactory. Therefore, there is still an unmet medical need for patients with ESCC. Here, we report the expression status of HDAC1 in human ESCC and matched paracancerous tissues, and the results indicated that HDAC1 was generally upregulated in ESCC specimens. Furthermore, we comprehensively assessed the anti-ESCC activity of a highly active HDAC1 inhibitor quisinostat. Quisinostat could effectively suppress cellular viability and proliferation of ESCC cells, as well as induce cell cycle arrest and apoptosis even at low treatment concentrations. The effectiveness was also observed in KYSE150 xenograft model when quisinostat was administered at tolerated doses (3 mg/kg and 10 mg/kg). Meanwhile, quisinostat also had the ability to suppress the migration and invasion (pivotal steps of tumor metastasis) of ESCC cells. Western blot analysis indicated that quisinostat exerted its anti-ESCC effects mainly through blockade of Akt/mTOR and MAPK/ERK signaling cascades. Overall, HDAC1 may serve as a potential therapeutic target for ESCC, and quisinostat deserves to be further assessed as a promising drug candidate for the treatment of ESCC.






Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2):87–108
Arnold M, Soerjomataram I, Ferlay J, Forman D (2015) Global incidence of oesophageal cancer by histological subtype in 2012. Gut 64(3):381–387
Liu FT, Gao H, Wu CW, Zhu ZM (2017) The association of plasma fibrinogen with clinicopathological features and prognosis in esophageal cancer patients. Oncotarget 8(54):93029–93038
Barret M, Prat F (2018) Diagnosis and treatment of superficial esophageal cancer. Ann Gastroenterol 31(3):256–265
Shaikh T, Meyer JE, Horwitz EM (2017) Optimal use of combined modality therapy in the treatment of esophageal cancer. Surg Oncol Clin N Am 26(3):405–429
Hsu PK (2018) The era of personalized multimodal treatments for esophageal cancer. Ann Transl Med 6(4):75
Goey AK, Sissung TM, Peer CJ, Figg WD (2016) Pharmacogenomics and histone deacetylase inhibitors. Pharmacogenomics 17(16):1807–1815
Qin HT, Li HQ, Liu F (2017) Selective histone deacetylase small molecule inhibitors: recent progress and perspectives. Expert Opin Ther Pat 27(5):621–636
Zibelman M, Wong YN, Devarajan K, Malizzia L, Corrigan A, Olszanski AJ, Denlinger CS, Roethke SK, Tetzlaff CH, Plimack ER (2015) Phase I study of the mTOR inhibitor ridaforolimus and the HDAC inhibitor vorinostat in advanced renal cell carcinoma and other solid tumors. Investig New Drugs 33(5):1040–1047
Wahaib K, Beggs AE, Campbell H, Kodali L, Ford PD (2016) Panobinostat: A histone deacetylase inhibitor for the treatment of relapsed or refractory multiple myeloma. Am J Health Syst Pharm 73(7):441–450
Yoon S, Eom GH (2016) HDAC and HDAC inhibitor: from cancer to cardiovascular diseases. Chonnam Med J 52(1):1–11
New M, Olzscha H, La Thangue NB (2012) HDAC inhibitor-based therapies: can we interpret the code? Mol Oncol 6(6):637–656
Song M, He G, Wang Y, Pang X, Zhang B (2016) Lentivirus-mediated knockdown of HDAC1 uncovers its role in esophageal cancer metastasis and chemosensitivity. J Cancer 7(12):1694–1700
Cao LL, Yue Z, Liu L, Pei L, Yin Y, Qin L, Zhao J, Liu H, Wang H, Jia M (2017) The expression of histone deacetylase HDAC1 correlates with the progression and prognosis of gastrointestinal malignancy. Oncotarget 8(24):39241–39253
Zhong L, Fu XY, Zou C, Yang LL, Zhou S, Yang J, Tang Y, Cheng C, Li LL, Xiang R, Chen LJ, Chen YZ, Wei YQ, Yang SY (2014) A preclinical evaluation of a novel multikinase inhibitor, SKLB-329, as a therapeutic agent against hepatocellular carcinoma. Int J Cancer 135(12):2972–2983
Conte M, De Palma R, Altucci L (2018) HDAC inhibitors as epigenetic regulators for cancer immunotherapy. Int J Biochem Cell Biol 98:65–74
Vicente-Dueñas C, Hauer J, Cobaleda C, Borkhardt A, Sánchez-García I (2018) Epigenetic priming in cancer initiation. Trends Cancer 4(6):408–417
Eckschlager T, Plch J, Stiborova M, Hrabeta J (2017) Histone deacetylase inhibitors as anticancer drugs. Int J Mol Sci 18(7):E1414
Muller BM, Jana L, Kasajima A, Lehmann A, Prinzler J, Budczies J, Winzer KJ, Dietel M, Weichert W, Denkert C (2013) Differential expression of histone deacetylases HDAC1, 2 and 3 in human breast cancer–overexpression of HDAC2 and HDAC3 is associated with clinicopathological indicators of disease progression. BMC Cancer 13:215
Colarossi L, Memeo L, Colarossi C, Aiello E, Iuppa A, Espina V, Liotta L, Mueller C (2014) Inhibition of histone deacetylase 4 increases cytotoxicity of docetaxel in gastric cancer cells. Proteomics Clin Appl 8(11–12):924–931
Arts J, King P, Mariën A, Floren W, Beliën A, Janssen L, Pilatte I, Roux B, Decrane L, Gilissen R, Hickson I, Vreys V, Cox E, Bol K, Talloen W, Goris I, Andries L, Du Jardin M, Janicot M, Page M, van Emelen K, Angibaud P (2009) JNJ-26481585, a novel "second-generation" oral histone deacetylase inhibitor, shows broad-spectrum preclinical antitumoral activity. Clin Cancer Res 15(22):6841–6851
Witt O, Sand K, Pekrun A (2000) Butyrate-induced erythroid differentiation of human K562 leukemia cells involves inhibition of ERK and activation of p38 MAP kinase pathways. Blood 95(7):2391–2396
Zhang P, Guo Z, Wu Y, Hu R, Du J, He X, Jiao X, Zhu X (2015) Histone deacetylase inhibitors inhibit the proliferation of gallbladder carcinoma cells by suppressing AKT/mTOR signaling. PLoS One 10(8):e0136193
Funding
This work was supported by the National Natural Science Foundation of China (81702286 and 81300882), the Fundamental Research Funds of Science & Technology Department of Sichuan Province (2017YSKY0001), and the Special Foundation for Young Scientists of Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital (2016QN11).
Author information
Authors and Affiliations
Contributions
L Zhong and S Zhou contributed equally to this article.
Conception and design: L Zhong, S Zhou, LY Su, and Q Peng;
Development of methodology: L Zhong, S Zhou, and RS Tong;
Acquisition of data: L Zhong, S Zhou, and JY Shi;
Analysis and interpretation of data: L Zhong, RS Tong, and Q Peng;
Technical and material supports: YX Zhu, L Bai, XM Duan, WZ Liu, and JK Bao; Writing and review of the manuscript: L Zhong, S Zhou, LY Su, and Q Peng;
Study supervision: RS Tong, LY Su, and Q Peng.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the Animal Care and Use Committee of Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital (Chengdu, Sichuan, China).
Rights and permissions
About this article
Cite this article
Zhong, L., Zhou, S., Tong, R. et al. Preclinical assessment of histone deacetylase inhibitor quisinostat as a therapeutic agent against esophageal squamous cell carcinoma. Invest New Drugs 37, 616–624 (2019). https://doi.org/10.1007/s10637-018-0651-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0651-4