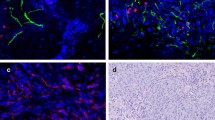
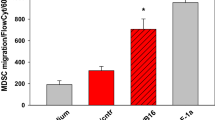
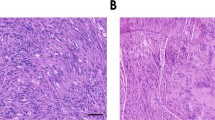
Abstract
Cancer cells are embedded within the tissue and interact dynamically with its components during cancer progression. Understanding the contribution of cellular components within the tumor microenvironment is crucial for the success of therapeutic applications. Here, we reveal the presence of perivascular GFAP+/Plp1+ cells within the tumor microenvironment. Using in vivo inducible Cre/loxP mediated systems, we demonstrated that these cells derive from tissue-resident Schwann cells. Genetic ablation of endogenous Schwann cells slowed down tumor growth and angiogenesis. Schwann cell-specific depletion also induced a boost in the immune surveillance by increasing tumor-infiltrating anti-tumor lymphocytes, while reducing immune-suppressor cells. In humans, a retrospective in silico analysis of tumor biopsies revealed that increased expression of Schwann cell-related genes within melanoma was associated with improved survival. Collectively, our study suggests that Schwann cells regulate tumor progression, indicating that manipulation of Schwann cells may provide a valuable tool to improve cancer patients’ outcomes.


















Similar content being viewed by others
Data availability
Data will be made available on reasonable request.
Abbreviations
- BP:
-
Biological processes
- BV:
-
Blood vessel
- CAFs:
-
Cancer-associated fibroblasts
- CD:
-
Cluster differentiation
- CEUA:
-
Ethics Animal Care and Use Committee
- CTLA-4:
-
Cytotoxic T lymphocyte Antigen-4
- DC:
-
Dendritic cells
- DEGs:
-
Differentially expressed genes
- DMEM:
-
Dulbecco's modified eagle medium
- DT:
-
Diphtheria toxin
- ES:
-
Effect size
- FBS:
-
Fetal bovine serum
- FSC-A:
-
Forward scatter area
- FSC-H:
-
Forward scatter height
- GFAP:
-
Glial fibrillary acidic protein
- GFP:
-
Green fluorescent protein
- GO:
-
Gene ontology
- iDTR:
-
Diphtheria toxin receptor
- IFN-γ:
-
Interferon gamma
- IL:
-
Interleukin
- NGFR:
-
Nerve growth factor receptor
- NG2:
-
Neuron-glial antigen 2
- NK:
-
Natural killer
- OCT:
-
Tissue-Tek
- p75:
-
Neurotrophin-75
- PBS:
-
Phosphate-buffered saline
- PD-1:
-
Programmed cell death protein 1
- PDGFRβ:
-
Platelet-derived growth factor receptor beta
- PFA:
-
Paraformaldehyde
- Plp1:
-
Proteolipid protein 1
- SC:
-
Schwann cell
- SKCM:
-
Skin cutaneous melanoma
- TCGA:
-
The cancer genome atlas
- TH:
-
Tyrosine hydroxylase
- TNBC:
-
Triple-negative breast cancer
- TUBB3:
-
Class III β tubulin
- UFMG:
-
Federal University of Minas Gerais
- UMAP:
-
Uniform Manifold Approximation and Projection
- WT:
-
Wild-type
- γδ:
-
Gamma Delta
- SEM:
-
Standard error
References
Catalano V, Turdo A, Di Franco S, Dieli F, Todaro M, Stassi G (2013) Tumor and its microenvironment: a synergistic interplay. Semin Cancer Biol 23(6 Pt B):522–532. https://doi.org/10.1016/j.semcancer.2013.08.007
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674. https://doi.org/10.1016/j.cell.2011.02.013
Swartz MA, Iida N, Roberts EW, Sangaletti S, Wong MH, Yull FE, Coussens LM, DeClerck YA (2012) Tumor microenvironment complexity: emerging roles in cancer therapy. Cancer Res 72(10):2473–2480. https://doi.org/10.1158/0008-5472.CAN-12-0122
Hanahan D, Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21(3):309–322. https://doi.org/10.1016/j.ccr.2012.02.022
Hinshaw DC, Shevde LA (2019) The tumor microenvironment innately modulates cancer progression. Cancer Res 79(18):4557–4566. https://doi.org/10.1158/0008-5472.CAN-18-3962
Senthebane DA, Rowe A, Thomford NE, Shipanga H, Munro D, Mazeedi M, Almazyadi HAM, Kallmeyer K, Dandara C, Pepper MS, Parker MI, Dzobo K (2017) The role of tumor microenvironment in chemoresistance: to survive, keep your enemies closer. Int J Mol Sci. https://doi.org/10.3390/ijms18071586
Renz BW, Takahashi R, Tanaka T, Macchini M, Hayakawa Y, Dantes Z, Maurer HC, Chen X, Jiang Z, Westphalen CB, Ilmer M, Valenti G, Mohanta SK, Habenicht AJR, Middelhoff M, Chu T, Nagar K, Tailor Y, Casadei R, Di Marco M, Kleespies A, Friedman RA, Remotti H, Reichert M, Worthley DL, Neumann J, Werner J, Iuga AC, Olive KP, Wang TC (2018) beta2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell 34(5):863–867. https://doi.org/10.1016/j.ccell.2018.10.010
Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, Frenette PS (2013) Autonomic nerve development contributes to prostate cancer progression. Science 341(6142):1236361. https://doi.org/10.1126/science.1236361
Zahalka AH, Arnal-Estape A, Maryanovich M, Nakahara F, Cruz CD, Finley LWS, Frenette PS (2017) Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 358(6361):321–326. https://doi.org/10.1126/science.aah5072
Prazeres P, Leonel C, Silva WN, Rocha BGS, Santos GSP, Costa AC, Picoli CC, Sena IFG, Goncalves WA, Vieira MS, Costa PAC, Campos L, Lopes MTP, Costa MR, Resende RR, Cunha TM, Mintz A, Birbrair A (2020) Ablation of sensory nerves favours melanoma progression. J Cell Mol Med. https://doi.org/10.1111/jcmm.15381
Zhao CM, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen CB, Andersen GT, Flatberg A, Johannessen H, Friedman RA, Renz BW, Sandvik AK, Beisvag V, Tomita H, Hara A, Quante M, Li Z, Gershon MD, Kaneko K, Fox JG, Wang TC, Chen D (2014) Denervation suppresses gastric tumorigenesis. Sci Transl Med 6(250):250ra115. https://doi.org/10.1126/scitranslmed.3009569
Saloman JL, Albers KM, Li D, Hartman DJ, Crawford HC, Muha EA, Rhim AD, Davis BM (2016) Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc Natl Acad Sci USA 113(11):3078–3083. https://doi.org/10.1073/pnas.1512603113
Renz BW, Takahashi R, Tanaka T, Macchini M, Hayakawa Y, Dantes Z, Maurer HC, Chen X, Jiang Z, Westphalen CB, Ilmer M, Valenti G, Mohanta SK, Habenicht AJR, Middelhoff M, Chu T, Nagar K, Tailor Y, Casadei R, Di Marco M, Kleespies A, Friedman RA, Remotti H, Reichert M, Worthley DL, Neumann J, Werner J, Iuga AC, Olive KP, Wang TC (2018) beta2 adrenergic-neurotrophin feed forward loop promotes pancreatic cancer. Cancer Cell 33(1):75–90. https://doi.org/10.1016/j.ccell.2017.11.007
Dubeykovskaya Z, Si Y, Chen X, Worthley DL, Renz BW, Urbanska AM, Hayakawa Y, Xu T, Westphalen CB, Dubeykovskiy A, Chen D, Friedman RA, Asfaha S, Nagar K, Tailor Y, Muthupalani S, Fox JG, Kitajewski J, Wang TC (2016) Neural innervation stimulates splenic TFF2 to arrest myeloid cell expansion and cancer. Nat Commun 7:10517. https://doi.org/10.1038/ncomms10517
Kamiya A, Hayama Y, Kato S, Shimomura A, Shimomura T, Irie K, Kaneko R, Yanagawa Y, Kobayashi K, Ochiya T (2019) Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat Neurosci 22(8):1289–1305. https://doi.org/10.1038/s41593-019-0430-3
Renz BW, Tanaka T, Sunagawa M, Takahashi R, Jiang Z, Macchini M, Dantes Z, Valenti G, White RA, Middelhoff MA, Ilmer M, Oberstein PE, Angele MK, Deng H, Hayakawa Y, Westphalen CB, Werner J, Remotti H, Reichert M, Tailor YH, Nagar K, Friedman RA, Iuga AC, Olive KP, Wang TC (2018) Cholinergic signaling via muscarinic receptors directly and indirectly suppresses pancreatic tumorigenesis and cancer stemness. Cancer Discov 8(11):1458–1473. https://doi.org/10.1158/2159-8290.CD-18-0046
Hayakawa Y, Sakitani K, Konishi M, Asfaha S, Niikura R, Tomita H, Renz BW, Tailor Y, Macchini M, Middelhoff M, Jiang Z, Tanaka T, Dubeykovskaya ZA, Kim W, Chen X, Urbanska AM, Nagar K, Westphalen CB, Quante M, Lin CS, Gershon MD, Hara A, Zhao CM, Chen D, Worthley DL, Koike K, Wang TC (2017) Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell 31(1):21–34. https://doi.org/10.1016/j.ccell.2016.11.005
Amit M, Takahashi H, Dragomir MP, Lindemann A, Gleber-Netto FO, Pickering CR, Anfossi S, Osman AA, Cai Y, Wang R, Knutsen E, Shimizu M, Ivan C, Rao X, Wang J, Silverman DA, Tam S, Zhao M, Caulin C, Zinger A, Tasciotti E, Dougherty PM, El-Naggar A, Calin GA, Myers JN (2020) Loss of p53 drives neuron reprogramming in head and neck cancer. Nature 578(7795):449–454. https://doi.org/10.1038/s41586-020-1996-3
Madeo M, Colbert PL, Vermeer DW, Lucido CT, Cain JT, Vichaya EG, Grossberg AJ, Muirhead D, Rickel AP, Hong Z, Zhao J, Weimer JM, Spanos WC, Lee JH, Dantzer R, Vermeer PD (2018) Cancer exosomes induce tumor innervation. Nat Commun 9(1):4284. https://doi.org/10.1038/s41467-018-06640-0
Gysler SM, Drapkin R (2021) Tumor innervation: peripheral nerves take control of the tumor microenvironment. J Clin Investig. https://doi.org/10.1172/JCI147276
Peng J, Chen H, Zhang B (2022) Nerve-stem cell crosstalk in skin regeneration and diseases. Trends Mol Med. https://doi.org/10.1016/j.molmed.2022.04.005
Picoli CC, Costa AC, Rocha BGS, Silva WN, Santos GSP, Prazeres P, Costa PAC, Oropeza A, da Silva RA, Azevedo VAC, Resende RR, Cunha TM, Mintz A, Birbrair A (2021) Sensory nerves in the spotlight of the stem cell niche. Stem Cells Transl Med 10(3):346–356. https://doi.org/10.1002/sctm.20-0284
Picoli CC, Goncalves BOP, Santos GSP, Rocha BGS, Costa AC, Resende RR, Birbrair A (2021) Pericytes cross-talks within the tumor microenvironment. Biochim Biophys Acta Rev Cancer 1876 2:188608. https://doi.org/10.1016/j.bbcan.2021.188608
Lousado L, Prazeres PHDM, Andreotti JP, Paiva AE, Azevedo PO, Santos GSP, Filev R, Mintz A, Birbrair A (2017) Schwann cell precursors as a source for adrenal gland chromaffin cells. Cell Death Dis 8:e3072
Scholz J, Woolf CJ (2007) The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 10(11):1361–1368. https://doi.org/10.1038/nn1992
Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ (2002) Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell 109(6):693–705
Li W, Kohara H, Uchida Y, James JM, Soneji K, Cronshaw DG, Zou YR, Nagasawa T, Mukouyama YS (2013) Peripheral nerve-derived CXCL12 and VEGF-A regulate the patterning of arterial vessel branching in developing limb skin. Dev Cell 24(4):359–371. https://doi.org/10.1016/j.devcel.2013.01.009
Colomar A, Robitaille R (2004) Glial modulation of synaptic transmission at the neuromuscular junction. Glia 47(3):284–289. https://doi.org/10.1002/glia.20086
De Logu F, Nassini R, Materazzi S, Carvalho Goncalves M, Nosi D, Rossi Degl’Innocenti D, Marone IM, Ferreira J, Li Puma S, Benemei S, Trevisan G, Monteiro S, de Araujo D, Patacchini R, Bunnett NW, Geppetti P (2017) Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice. Nat Commun 8(1):1887. https://doi.org/10.1038/s41467-017-01739-2
Rinwa P, Calvo-Enrique L, Zhang MD, Nyengaard JR, Karlsson P, Ernfors P (2021) Demise of nociceptive Schwann cells causes nerve retraction and pain hyperalgesia. Pain 162(6):1816–1827. https://doi.org/10.1097/j.pain.0000000000002169
Wei Z, Fei Y, Su W, Chen G (2019) Emerging role of Schwann cells in neuropathic pain: receptors, glial mediators and myelination. Front Cell Neurosci 13:116. https://doi.org/10.3389/fncel.2019.00116
Wekerle H, Schwab M, Linington C, Meyermann R (1986) Antigen presentation in the peripheral nervous system: Schwann cells present endogenous myelin autoantigens to lymphocytes. Eur J Immunol 16(12):1551–1557. https://doi.org/10.1002/eji.1830161214
Martin I, Nguyen TD, Krell V, Greiner JF, Muller J, Hauser S, Heimann P, Widera D (2012) Generation of Schwann cell-derived multipotent neurospheres isolated from intact sciatic nerve. Stem Cell Rev 8(4):1178–1187. https://doi.org/10.1007/s12015-012-9387-2
Widera D, Heimann P, Zander C, Imielski Y, Heidbreder M, Heilemann M, Kaltschmidt C, Kaltschmidt B (2011) Schwann cells can be reprogrammed to multipotency by culture. Stem Cells Dev 20(12):2053–2064. https://doi.org/10.1089/scd.2010.0525
Masaki T, Qu J, Cholewa-Waclaw J, Burr K, Raaum R, Rambukkana A (2013) Reprogramming adult Schwann cells to stem cell-like cells by leprosy bacilli promotes dissemination of infection. Cell 152(1–2):51–67. https://doi.org/10.1016/j.cell.2012.12.014
Kaukua N, Shahidi MK, Konstantinidou C, Dyachuk V, Kaucka M, Furlan A, An Z, Wang L, Hultman I, Ahrlund-Richter L, Blom H, Brismar H, Lopes NA, Pachnis V, Suter U, Clevers H, Thesleff I, Sharpe P, Ernfors P, Fried K, Adameyko I (2014) Glial origin of mesenchymal stem cells in a tooth model system. Nature 513(7519):551–554. https://doi.org/10.1038/nature13536
Adameyko I, Lallemend F, Aquino JB, Pereira JA, Topilko P, Muller T, Fritz N, Beljajeva A, Mochii M, Liste I, Usoskin D, Suter U, Birchmeier C, Ernfors P (2009) Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell 139(2):366–379. https://doi.org/10.1016/j.cell.2009.07.049
Uesaka T, Nagashimada M, Enomoto H (2015) Neuronal differentiation in schwann cell lineage underlies postnatal neurogenesis in the enteric nervous system. J Neurosci 35(27):9879–9888. https://doi.org/10.1523/JNEUROSCI.1239-15.2015
Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, Nakauchi H (2011) Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 147(5):1146–1158. https://doi.org/10.1016/j.cell.2011.09.053
Silva WN, Leonel C, Prazeres PHDM, Sena IFG, Guerra DAP, Heller D, Diniz IMA, Fortuna V, Mintz A, Birbrair A (2018) Role of Schwann cells in cutaneous wound healing. Wound Repair Regen 26(5):392–397. https://doi.org/10.1111/wrr.12647
Carr MJ, Johnston AP (2017) Schwann cells as drivers of tissue repair and regeneration. Curr Opin Neurobiol 47:52–57. https://doi.org/10.1016/j.conb.2017.09.003
Joseph NM, Mosher JT, Buchstaller J, Snider P, McKeever PE, Lim M, Conway SJ, Parada LF, Zhu Y, Morrison SJ (2008) The loss of Nf1 transiently promotes self-renewal but not tumorigenesis by neural crest stem cells. Cancer Cell 13(2):129–140. https://doi.org/10.1016/j.ccr.2008.01.003
Roosli C, Linthicum FH Jr, Cureoglu S, Merchant SN (2012) What is the site of origin of cochleovestibular schwannomas? Audiol Neurootol 17(2):121–125. https://doi.org/10.1159/000331394
Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS (2019) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol 21(Suppl 5):v1–v100. https://doi.org/10.1093/neuonc/noz150
Furlan A, Dyachuk V, Kastriti ME, Calvo-Enrique L, Abdo H, Hadjab S, Chontorotzea T, Akkuratova N, Usoskin D, Kamenev D, Petersen J, Sunadome K, Memic F, Marklund U, Fried K, Topilko P, Lallemend F, Kharchenko PV, Ernfors P, Adameyko I (2017) Multipotent peripheral glial cells generate neuroendocrine cells of the adrenal medulla. Science. https://doi.org/10.1126/science.aal3753
Swanson BJ, McDermott KM, Singh PK, Eggers JP, Crocker PR, Hollingsworth MA (2007) MUC1 is a counter-receptor for myelin-associated glycoprotein (Siglec-4a) and their interaction contributes to adhesion in pancreatic cancer perineural invasion. Cancer Res 67(21):10222–10229. https://doi.org/10.1158/0008-5472.CAN-06-2483
Deborde S, Omelchenko T, Lyubchik A, Zhou Y, He S, McNamara WF, Chernichenko N, Lee SY, Barajas F, Chen CH, Bakst RL, Vakiani E, He S, Hall A, Wong RJ (2016) Schwann cells induce cancer cell dispersion and invasion. J Clin Investig 126(4):1538–1554. https://doi.org/10.1172/JCI82658
Sroka IC, Chopra H, Das L, Gard JM, Nagle RB, Cress AE (2016) Schwann cells increase prostate and pancreatic tumor cell invasion using laminin binding A6 integrin. J Cell Biochem 117(2):491–499. https://doi.org/10.1002/jcb.25300
Shurin GV, Kruglov O, Ding F, Lin Y, Hao X, Keskinov AA, You Z, Lokshin AE, LaFramboise WA, Falo LD Jr, Shurin MR, Bunimovich YL (2019) Melanoma-induced reprogramming of schwann cell signaling aids tumor growth. Cancer Res 79(10):2736–2747. https://doi.org/10.1158/0008-5472.CAN-18-3872
Snippert HJ, Clevers H (2011) Tracking adult stem cells. EMBO Rep 12(2):113–122. https://doi.org/10.1038/embor.2010.216
Birbrair A, Borges IDT, Gilson Sena IF, Almeida GG, da Silva ML, Goncalves R, Mintz A, Delbono O (2017) How plastic are pericytes? Stem Cells Dev 26(14):1013–1019. https://doi.org/10.1089/scd.2017.0044
Khan JA, Mendelson A, Kunisaki Y, Birbrair A, Kou Y, Arnal-Estape A, Pinho S, Ciero P, Nakahara F, Ma’ayan A, Bergman A, Merad M, Frenette PS (2016) Fetal liver hematopoietic stem cell niches associate with portal vessels. Science 351(6269):176–180. https://doi.org/10.1126/science.aad0084
Asada N, Kunisaki Y, Pierce H, Wang Z, Fernandez NF, Birbrair A, Ma’ayan A, Frenette PS (2017) Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol 19(3):214–223. https://doi.org/10.1038/ncb3475
Nobre AR, Risson E, Singh DK, Martino JD, Cheung JF, Wang J, Johnson J, Russnes HG, Bravo-Cordero JJ, Birbrair A, Naume B, Azhar M, Frenette PS, Aguirre-Ghiso JA (2021) Bone marrow NG2+/Nestin+ mesenchymal stem cells drive DTC dormancy via TGFβ2. Nat Cancer 2:327–339
Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I (1999) Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res 8(4):265–277. https://doi.org/10.1023/a:1008942828960
Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW (2011) CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475(7355):222–225. https://doi.org/10.1038/nature10138
Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H (2010) A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13(1):133–140. https://doi.org/10.1038/nn.2467
Birbrair A, Zhang T, Files DC, Mannava S, Smith T, Wang ZM, Messi ML, Mintz A, Delbono O (2014) Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem Cell Res Ther 5(6):122. https://doi.org/10.1186/scrt512
Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O (2013) Skeletal muscle neural progenitor cells exhibit properties of NG2-glia. Exp Cell Res 319(1):45–63. https://doi.org/10.1016/j.yexcr.2012.09.008
Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O (2013) Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev 22(16):2298–2314. https://doi.org/10.1089/scd.2012.0647
Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O (2013) Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. Am J Physiol Cell Physiol 305(11):C1098–C1113. https://doi.org/10.1152/ajpcell.00171.2013
Gomes NA, do Valle IB, Gleber-Netto FO, Silva TA, Oliveira HMdC, de Oliveira RF, Ferreira LdAQ, Castilho LS, Reis PHRG, Prazeres PHDM, Menezes GB, de Magalhães CS, Mesquita RA, Marques MM, Birbrair A, Diniz IMA (2022) Nestin and NG2 transgenes reveal two populations of perivascular cells stimulated by photobiomodulation. J Cell Physiol 237:2198–2210. https://doi.org/10.1002/jcp.30680
do Valle IB, Prazeres PHDM, Mesquita RA, Silva TA, de Castro Oliveira HM, Castro PR, Freitas IDP, Oliveira SR, Gomes NA, de Oliveira RF, Marquiore LF, Macari S, do Amaral FA, Jácome-Santos H, Barcelos LS, Menezes GB, Marques MM, Birbrair A, Diniz IMA (2010) Photobiomodulation drives pericyte mobilization towards skin regeneration. Sci Rep 10(1):19257. https://doi.org/10.1038/s41598-020-76243-7
Doerflinger NH, Macklin WB, Popko B (2003) Inducible site-specific recombination in myelinating cells. Genesis 35(1):63–72. https://doi.org/10.1002/gene.10154
Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, Jung S, Waisman A (2005) A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods 2(6):419–426. https://doi.org/10.1038/nmeth762
Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, Thomas GV, Sawyers CL (2003) Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell 4(3):223–238. https://doi.org/10.1016/s1535-6108(03)00197-1
Birbrair A, Sattiraju A, Zhu D, Zulato G, Batista I, Nguyen VT, Messi ML, Solingapuram Sai KK, Marini FC, Delbono O, Mintz A (2017) Novel peripherally derived neural-like stem cells as therapeutic carriers for treating glioblastomas. Stem Cells Transl Med 6(2):471–481. https://doi.org/10.5966/sctm.2016-0007
Birbrair A, Wang ZM, Messi ML, Enikolopov GN, Delbono O (2011) Nestin-GFP transgene reveals neural precursor cells in adult skeletal muscle. PLoS One 6(2):e16816. https://doi.org/10.1371/journal.pone.0016816
Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G (2004) Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol 469(3):311–324. https://doi.org/10.1002/cne.10964
Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM (1997) Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res 57(16):3325–3330
Liu Z, Eltoum IE, Guo B, Beck BH, Cloud GA, Lopez RD (2008) Protective immunosurveillance and therapeutic antitumor activity of gammadelta T cells demonstrated in a mouse model of prostate cancer. J Immunol 180(9):6044–6053. https://doi.org/10.4049/jimmunol.180.9.6044
Yeon A, Wang Y, Su S, Lo EM, Kim HL (2020) Syngeneic murine model for prostate cancer using RM1 cells transfected with gp100. Prostate 80(5):424–431. https://doi.org/10.1002/pros.23957
Coimbra-Campos LMC, Silva WN, Baltazar LM, Costa PAC, Prazeres PHDM, Picoli CC, Costa AC, Rocha BGS, Santos GSP, Oliveira FMS, Pinto MCX, Amorim JH, Azevedo VAC, Souza DG, Russo RC, Resende RR, Mintz A, Birbrair A (2021) Circulating Nestin-GFP+ cells participate in the pathogenesis of Paracoccidioides brasiliensis in the lungs. Stem Cell Rev Rep 17:1874
Bohn T, Rapp S, Luther N, Klein M, Bruehl TJ, Kojima N, Aranda Lopez P, Hahlbrock J, Muth S, Endo S, Pektor S, Brand A, Renner K, Popp V, Gerlach K, Vogel D, Lueckel C, Arnold-Schild D, Pouyssegur J, Kreutz M, Huber M, Koenig J, Weigmann B, Probst HC, von Stebut E, Becker C, Schild H, Schmitt E, Bopp T (2018) Tumor immunoevasion via acidosis-dependent induction of regulatory tumor-associated macrophages. Nat Immunol 19(12):1319–1329. https://doi.org/10.1038/s41590-018-0226-8
Gast CE, Silk AD, Zarour L, Riegler L, Burkhart JG, Gustafson KT, Parappilly MS, Roh-Johnson M, Goodman JR, Olson B, Schmidt M, Swain JR, Davies PS, Shasthri V, Iizuka S, Flynn P, Watson S, Korkola J, Courtneidge SA, Fischer JM, Jaboin J, Billingsley KG, Lopez CD, Burchard J, Gray J, Coussens LM, Sheppard BC, Wong MH (2018) Cell fusion potentiates tumor heterogeneity and reveals circulating hybrid cells that correlate with stage and survival. Sci Adv 4(9):eaat7828. https://doi.org/10.1126/sciadv.aat7828
Herring BP, Hoggatt AM, Burlak C, Offermanns S (2014) Previously differentiated medial vascular smooth muscle cells contribute to neointima formation following vascular injury. Vasc Cell 6:21. https://doi.org/10.1186/2045-824X-6-21
Nikolakopoulou AM, Montagne A, Kisler K, Dai Z, Wang Y, Huuskonen MT, Sagare AP, Lazic D, Sweeney MD, Kong P, Wang M, Owens NC, Lawson EJ, Xie X, Zhao Z, Zlokovic BV (2019) Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat Neurosci 22(7):1089–1098. https://doi.org/10.1038/s41593-019-0434-z
Cha JH, Chang MY, Richardson JA, Eidels L (2003) Transgenic mice expressing the diphtheria toxin receptor are sensitive to the toxin. Mol Microbiol 49(1):235–240. https://doi.org/10.1046/j.1365-2958.2003.03550.x
Leclerc M, Voilin E, Gros G, Corgnac S, de Montpreville V, Validire P, Bismuth G, Mami-Chouaib F (2019) Regulation of antitumour CD8 T-cell immunity and checkpoint blockade immunotherapy by Neuropilin-1. Nat Commun 10(1):3345. https://doi.org/10.1038/s41467-019-11280-z
Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H (1984) Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 133(4):1710–1715
Sena IFG, Rocha BGS, Picoli CC, Santos GSP, Costa AC, Goncalves BOP, Garcia APV, Soltani-Asl M, Coimbra-Campos LMC, Silva WN, Costa PAC, Pinto MCX, Amorim JH, Azevedo VAC, Resende RR, Heller D, Cassali GD, Mintz A, Birbrair A (2021) C(3)1-TAg in C57BL/6 J background as a model to study mammary tumor development. Histochem Cell Biol. https://doi.org/10.1007/s00418-021-01995-w
Sena IFG, Fernandes LL, Lorandi LL, Santana TV, Cintra L, Lima IF, Iwai LK, Kramer JM, Birbrair A, Heller D (2022) Identification of early biomarkers in saliva in genetically engineered mouse model C(3)1-TAg of breast cancer. Sci Rep. https://doi.org/10.1038/s41598-022-14514-1
Newman AM, Steen CB, Liu CL, Gentles AJ, Chaudhuri AA, Scherer F, Khodadoust MS, Esfahani MS, Luca BA, Steiner D, Diehn M, Alizadeh AA (2019) Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat Biotechnol 37(7):773–782. https://doi.org/10.1038/s41587-019-0114-2
Wolbert J, Li X, Heming M, Mausberg AK, Akkermann D, Frydrychowicz C, Fledrich R, Groeneweg L, Schulz C, Stettner M, Alonso Gonzalez N, Wiendl H, Stassart R, Meyer Zu Horste G (2020) Redefining the heterogeneity of peripheral nerve cells in health and autoimmunity. Proc Natl Acad Sci USA 117(17):9466–9476. https://doi.org/10.1073/pnas.1912139117
Tasdemir-Yilmaz OE, Druckenbrod NR, Olukoya OO, Dong W, Yung AR, Bastille I, Pazyra-Murphy MF, Sitko AA, Hale EB, Vigneau S, Gimelbrant AA, Kharchenko PV, Goodrich LV, Segal RA (2021) Diversity of developing peripheral glia revealed by single-cell RNA sequencing. Dev Cell 56(17):2516–2535. https://doi.org/10.1016/j.devcel.2021.08.005
Liu Z, Jin YQ, Chen L, Wang Y, Yang X, Cheng J, Wu W, Qi Z, Shen Z (2015) Specific marker expression and cell state of Schwann cells during culture in vitro. PLoS ONE 10(4):e0123278. https://doi.org/10.1371/journal.pone.0123278
Peng K, Sant D, Andersen N, Silvera R, Camarena V, Pinero G, Graham R, Khan A, Xu XM, Wang G, Monje PV (2020) Magnetic separation of peripheral nerve-resident cells underscores key molecular features of human Schwann cells and fibroblasts: an immunochemical and transcriptomics approach. Sci Rep 10(1):18433. https://doi.org/10.1038/s41598-020-74128-3
Skelly DA, Squiers GT, McLellan MA, Bolisetty MT, Robson P, Rosenthal NA, Pinto AR (2018) Single-cell transcriptional profiling reveals cellular diversity and intercommunication in the mouse heart. Cell Rep 22(3):600–610. https://doi.org/10.1016/j.celrep.2017.12.072
Clements MP, Byrne E, Camarillo Guerrero LF, Cattin AL, Zakka L, Ashraf A, Burden JJ, Khadayate S, Lloyd AC, Marguerat S, Parrinello S (2017) The wound microenvironment reprograms Schwann cells to invasive mesenchymal-like cells to drive peripheral nerve regeneration. Neuron 96(1):98–114. https://doi.org/10.1016/j.neuron.2017.09.008
Parmantier E, Lynn B, Lawson D, Turmaine M, Namini SS, Chakrabarti L, McMahon AP, Jessen KR, Mirsky R (1999) Schwann cell-derived Desert hedgehog controls the development of peripheral nerve sheaths. Neuron 23(4):713–724. https://doi.org/10.1016/s0896-6273(01)80030-1
Woods C, Kapur RP, Bischoff A, Lovell M, Arnold M, Pena A, Flockton A, Sharkey KA, Belkind-Gerson J (2021) Neurons populating the rectal extrinsic nerves in humans express neuronal and Schwann cell markers. Neurogastroenterol Motil 33(7):e14074. https://doi.org/10.1111/nmo.14074
Frank M, Schaeren-Wiemers N, Schneider R, Schwab ME (1999) Developmental expression pattern of the myelin proteolipid MAL indicates different functions of MAL for immature Schwann cells and in a late step of CNS myelinogenesis. J Neurochem 73(2):587–597. https://doi.org/10.1046/j.1471-4159.1999.0730587.x
Torres-Mejia E, Trumbach D, Kleeberger C, Dornseifer U, Orschmann T, Backer T, Brenke JK, Hadian K, Wurst W, Lopez-Schier H, Desbordes SC (2020) Sox2 controls Schwann cell self-organization through fibronectin fibrillogenesis. Sci Rep 10(1):1984. https://doi.org/10.1038/s41598-019-56877-y
Mazzara PG, Massimino L, Pellegatta M, Ronchi G, Ricca A, Iannielli A, Giannelli SG, Cursi M, Cancellieri C, Sessa A, Del Carro U, Quattrini A, Geuna S, Gritti A, Taveggia C, Broccoli V (2017) Two factor-based reprogramming of rodent and human fibroblasts into Schwann cells. Nat Commun 8:14088. https://doi.org/10.1038/ncomms14088
Frob F, Sock E, Tamm ER, Saur AL, Hillgartner S, Williams TJ, Fujii T, Fukunaga R, Wegner M (2019) Ep400 deficiency in Schwann cells causes persistent expression of early developmental regulators and peripheral neuropathy. Nat Commun 10(1):2361. https://doi.org/10.1038/s41467-019-10287-w
Doddrell RD, Dun XP, Moate RM, Jessen KR, Mirsky R, Parkinson DB (2012) Regulation of Schwann cell differentiation and proliferation by the Pax-3 transcription factor. Glia 60(9):1269–1278. https://doi.org/10.1002/glia.22346
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13(11):2498–2504. https://doi.org/10.1101/gr.1239303
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ, Mering CV (2019) STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 47(D1):D607–D613. https://doi.org/10.1093/nar/gky1131
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15(12):550. https://doi.org/10.1186/s13059-014-0550-8
Ge SX, Jung D, Yao R (2020) ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics 36(8):2628–2629. https://doi.org/10.1093/bioinformatics/btz931
Supek F, Bosnjak M, Skunca N, Smuc T (2011) REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE 6(7):e21800. https://doi.org/10.1371/journal.pone.0021800
Chen S, Zhu G, Yang Y, Wang F, Xiao YT, Zhang N, Bian X, Zhu Y, Yu Y, Liu F, Dong K, Mariscal J, Liu Y, Soares F, Loo Yau H, Zhang B, Chen W, Wang C, Chen D, Guo Q, Yi Z, Liu M, Fraser M, De Carvalho DD, Boutros PC, Di Vizio D, Jiang Z, van der Kwast T, Berlin A, Wu S, Wang J, He HH, Ren S (2021) Single-cell analysis reveals transcriptomic remodellings in distinct cell types that contribute to human prostate cancer progression. Nat Cell Biol 23(1):87–98. https://doi.org/10.1038/s41556-020-00613-6
Wu SZ, Roden DL, Wang C, Holliday H, Harvey K, Cazet AS, Murphy KJ, Pereira B, Al-Eryani G, Bartonicek N, Hou R, Torpy JR, Junankar S, Chan CL, Lam CE, Hui MN, Gluch L, Beith J, Parker A, Robbins E, Segara D, Mak C, Cooper C, Warrier S, Forrest A, Powell J, O’Toole S, Cox TR, Timpson P, Lim E, Liu XS, Swarbrick A (2020) Stromal cell diversity associated with immune evasion in human triple-negative breast cancer. EMBO J 39(19):e104063. https://doi.org/10.15252/embj.2019104063
Tirosh I, Izar B, Prakadan SM, Wadsworth MH 2nd, Treacy D, Trombetta JJ, Rotem A, Rodman C, Lian C, Murphy G, Fallahi-Sichani M, Dutton-Regester K, Lin JR, Cohen O, Shah P, Lu D, Genshaft AS, Hughes TK, Ziegler CG, Kazer SW, Gaillard A, Kolb KE, Villani AC, Johannessen CM, Andreev AY, Van Allen EM, Bertagnolli M, Sorger PK, Sullivan RJ, Flaherty KT, Frederick DT, Jane-Valbuena J, Yoon CH, Rozenblatt-Rosen O, Shalek AK, Regev A, Garraway LA (2016) Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352(6282):189–196. https://doi.org/10.1126/science.aad0501
Fritz CO, Morris PE, Richler JJ (2012) Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen 141(1):2–18. https://doi.org/10.1037/a0024338
Hopkins WG, Marshall SW, Batterham AM, Hanin J (2009) Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc 41(1):3–13. https://doi.org/10.1249/MSS.0b013e31818cb278
Erin N, Zhao W, Bylander J, Chase G, Clawson G (2006) Capsaicin-induced inactivation of sensory neurons promotes a more aggressive gene expression phenotype in breast cancer cells. Breast Cancer Res Treat 99(3):351–364. https://doi.org/10.1007/s10549-006-9219-7
Costa PAC, Silva WN, Prazeres P, Picoli CC, Guardia GDA, Costa AC, Oliveira MA, Guimaraes PPG, Goncalves R, Pinto MCX, Amorim JH, Azevedo VAC, Resende RR, Russo RC, Cunha TM, Galante PAF, Mintz A, Birbrair A (2021) Chemogenetic modulation of sensory neurons reveals their regulating role in melanoma progression. Acta Neuropathol Commun 9(1):183. https://doi.org/10.1186/s40478-021-01273-9
Jessen KR, Mirsky R, Lloyd AC (2015) Schwann cells: development and role in nerve repair. Cold Spring Harb Perspect Biol 7(7):a020487. https://doi.org/10.1101/cshperspect.a020487
Bechmann I, Priller J, Kovac A, Bontert M, Wehner T, Klett FF, Bohsung J, Stuschke M, Dirnagl U, Nitsch R (2001) Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur J Neurosci 14(10):1651–1658
Guillemin GJ, Brew BJ (2004) Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukoc Biol 75(3):388–397. https://doi.org/10.1189/jlb.0303114
Honda M, Surewaard BGJ, Watanabe M, Hedrick CC, Lee WY, Brown K, McCoy KD, Kubes P (2020) Perivascular localization of macrophages in the intestinal mucosa is regulated by Nr4a1 and the microbiome. Nat Commun 11(1):1329. https://doi.org/10.1038/s41467-020-15068-4
Mai CL, Tan Z, Xu YN, Zhang JJ, Huang ZH, Wang D, Zhang H, Gui WS, Zhang J, Lin ZJ, Meng YT, Wei X, Jie YT, Grace PM, Wu LJ, Zhou LJ, Liu XG (2021) CXCL12-mediated monocyte transmigration into brain perivascular space leads to neuroinflammation and memory deficit in neuropathic pain. Theranostics 11(3):1059–1078. https://doi.org/10.7150/thno.44364
Roubeix C, Dominguez E, Raoul W, Guillonneau X, Paques M, Sahel JA, Sennlaub F (2019) Mo-derived perivascular macrophage recruitment protects against endothelial cell death in retinal vein occlusion. J Neuroinflamm 16(1):157. https://doi.org/10.1186/s12974-019-1547-8
Schnieder TP, Zhou Qin ID, Trencevska-Ivanovska I, Rosoklija G, Stankov A, Pavlovski G, Mann JJ, Dwork AJ (2019) Blood vessels and perivascular phagocytes of prefrontal white and gray matter in suicide. J Neuropathol Exp Neurol 78(1):15–30. https://doi.org/10.1093/jnen/nly103
Jolivel V, Bicker F, Biname F, Ploen R, Keller S, Gollan R, Jurek B, Birkenstock J, Poisa-Beiro L, Bruttger J, Opitz V, Thal SC, Waisman A, Bauerle T, Schafer MK, Zipp F, Schmidt MHH (2015) Perivascular microglia promote blood vessel disintegration in the ischemic penumbra. Acta Neuropathol 129(2):279–295. https://doi.org/10.1007/s00401-014-1372-1
Koizumi T, Taguchi K, Mizuta I, Toba H, Ohigashi M, Onishi O, Ikoma K, Miyata S, Nakata T, Tanaka M, Foulquier S, Steinbusch HWM, Mizuno T (2019) Transiently proliferating perivascular microglia harbor M1 type and precede cerebrovascular changes in a chronic hypertension model. J Neuroinflamm 16(1):79. https://doi.org/10.1186/s12974-019-1467-7
Hamann J, Koning N, Pouwels W, Ulfman LH, van Eijk M, Stacey M, Lin HH, Gordon S, Kwakkenbos MJ (2007) EMR1, the human homolog of F4/80, is an eosinophil-specific receptor. Eur J Immunol 37(10):2797–2802. https://doi.org/10.1002/eji.200737553
Mii S, Uehara F, Yano S, Tran B, Miwa S, Hiroshima Y, Amoh Y, Katsuoka K, Hoffman RM (2013) Nestin-expressing stem cells promote nerve growth in long-term 3-dimensional gelfoam(R)-supported histoculture. PLoS ONE 8(6):e67153. https://doi.org/10.1371/journal.pone.0067153
Kang H, Tian L, Son YJ, Zuo Y, Procaccino D, Love F, Hayworth C, Trachtenberg J, Mikesh M, Sutton L, Ponomareva O, Mignone J, Enikolopov G, Rimer M, Thompson W (2007) Regulation of the intermediate filament protein nestin at rodent neuromuscular junctions by innervation and activity. J Neurosci 27(22):5948–5957. https://doi.org/10.1523/JNEUROSCI.0621-07.2007
Kang H, Tian L, Mikesh M, Lichtman JW, Thompson WJ (2014) Terminal Schwann cells participate in neuromuscular synapse remodeling during reinnervation following nerve injury. J Neurosci 34(18):6323–6333. https://doi.org/10.1523/JNEUROSCI.4673-13.2014
Isern J, Garcia-Garcia A, Martin AM, Arranz L, Martin-Perez D, Torroja C, Sanchez-Cabo F, Mendez-Ferrer S (2014) The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. Elife 3:e03696. https://doi.org/10.7554/eLife.03696
Goncalves NP, Jager SE, Richner M, Murray SS, Mohseni S, Jensen TS, Vaegter CB (2020) Schwann cell p75 neurotrophin receptor modulates small fiber degeneration in diabetic neuropathy. Glia 68(12):2725–2743. https://doi.org/10.1002/glia.23881
Tomita K, Kubo T, Matsuda K, Fujiwara T, Yano K, Winograd JM, Tohyama M, Hosokawa K (2007) The neurotrophin receptor p75NTR in Schwann cells is implicated in remyelination and motor recovery after peripheral nerve injury. Glia 55(11):1199–1208. https://doi.org/10.1002/glia.20533
Mora J, Cheung NK, Juan G, Illei P, Cheung I, Akram M, Chi S, Ladanyi M, Cordon-Cardo C, Gerald WL (2001) Neuroblastic and Schwannian stromal cells of neuroblastoma are derived from a tumoral progenitor cell. Cancer Res 61(18):6892–6898
Bourdeaut F, Ribeiro A, Paris R, Pierron G, Couturier J, Peuchmaur M, Delattre O (2008) In neuroblastic tumours, Schwann cells do not harbour the genetic alterations of neuroblasts but may nevertheless share the same clonal origin. Oncogene 27(21):3066–3071. https://doi.org/10.1038/sj.onc.1210965
Iyengar B, Singh AV (2010) Patterns of neural differentiation in melanomas. J Biomed Sci 17:87. https://doi.org/10.1186/1423-0127-17-87
Banerjee SS, Eyden B (2008) Divergent differentiation in malignant melanomas: a review. Histopathology 52(2):119–129. https://doi.org/10.1111/j.1365-2559.2007.02823.x
Van Raamsdonk CD, Deo M (2013) Links between Schwann cells and melanocytes in development and disease. Pigment Cell Melanoma Res 26(5):634–645. https://doi.org/10.1111/pcmr.12134
Venneri MA, De Palma M, Ponzoni M, Pucci F, Scielzo C, Zonari E, Mazzieri R, Doglioni C, Naldini L (2007) Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood 109(12):5276–5285. https://doi.org/10.1182/blood-2006-10-053504
Yang M, McKay D, Pollard JW, Lewis CE (2018) Diverse functions of macrophages in different tumor microenvironments. Cancer Res 78(19):5492–5503. https://doi.org/10.1158/0008-5472.CAN-18-1367
Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, Friedman R, Varro A, Tycko B, Wang TC (2011) Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 19(2):257–272. https://doi.org/10.1016/j.ccr.2011.01.020
Klopp AH, Spaeth EL, Dembinski JL, Woodward WA, Munshi A, Meyn RE, Cox JD, Andreeff M, Marini FC (2007) Tumor irradiation increases the recruitment of circulating mesenchymal stem cells into the tumor microenvironment. Cancer Res 67(24):11687–11695. https://doi.org/10.1158/0008-5472.CAN-07-1406
Hosaka K, Yang Y, Seki T, Fischer C, Dubey O, Fredlund E, Hartman J, Religa P, Morikawa H, Ishii Y, Sasahara M, Larsson O, Cossu G, Cao R, Lim S, Cao Y (2016) Pericyte-fibroblast transition promotes tumor growth and metastasis. Proc Natl Acad Sci USA 113(38):E5618-5627. https://doi.org/10.1073/pnas.1608384113
Cooke VG, LeBleu VS, Keskin D, Khan Z, O’Connell JT, Teng Y, Duncan MB, Xie L, Maeda G, Vong S, Sugimoto H, Rocha RM, Damascena A, Brentani RR, Kalluri R (2012) Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell 21(1):66–81. https://doi.org/10.1016/j.ccr.2011.11.024
Birbrair A, Zhang T, Wang ZM, Messi ML, Olson JD, Mintz A, Delbono O (2014) Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol 307(1):C25-38. https://doi.org/10.1152/ajpcell.00084.2014
Sinha D, Chong L, George J, Schluter H, Monchgesang S, Mills S, Li J, Parish C, Bowtell D, Kaur P, Australian Ovarian Cancer Study G (2016) Pericytes promote malignant ovarian cancer progression in mice and predict poor prognosis in serous ovarian cancer patients. Clin Cancer Res 22(7):1813–1824. https://doi.org/10.1158/1078-0432.CCR-15-1931
Eilken HM, Dieguez-Hurtado R, Schmidt I, Nakayama M, Jeong HW, Arf H, Adams S, Ferrara N, Adams RH (2017) Pericytes regulate VEGF-induced endothelial sprouting through VEGFR1. Nat Commun 8(1):1574. https://doi.org/10.1038/s41467-017-01738-3
Bernardes SS, Pinto MCX, Amorim JH, Azevedo VAC, Resende RR, Mintz A, Birbrair A (2022) Glioma pericytes promote angiogenesis by producing periostin. Cell Mol Neurobiol 42(3):557–564. https://doi.org/10.1007/s10571-020-00975-3
Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O (2015) Pericytes at the intersection between tissue regeneration and pathology. Clin Sci (Lond) 128(2):81–93. https://doi.org/10.1042/CS20140278
Paiva AE, Lousado L, Guerra DAP, Azevedo PO, Sena IFG, Andreotti JP, Santos GSP, Goncalves R, Mintz A, Birbrair A (2018) Pericytes in the premetastatic niche. Cancer Res 78(11):2779–2786. https://doi.org/10.1158/0008-5472.CAN-17-3883
Sena IFG, Paiva AE, Prazeres P, Azevedo PO, Lousado L, Bhutia SK, Salmina AB, Mintz A, Birbrair A (2018) Glioblastoma-activated pericytes support tumor growth via immunosuppression. Cancer Med 7(4):1232–1239. https://doi.org/10.1002/cam4.1375
Valle IB, Schuch LF, da Silva JM, Gala-Garcia A, Diniz IMA, Birbrair A, Abreu LG, Silva TA (2020) Pericyte in oral squamous cell carcinoma: a systematic review. Head Neck Pathol 14(4):1080–1091. https://doi.org/10.1007/s12105-020-01188-2
Guerra DAP, Paiva AE, Sena IFG, Azevedo PO, Silva WN, Mintz A, Birbrair A (2018) Targeting glioblastoma-derived pericytes improves chemotherapeutic outcome. Angiogenesis 21(4):667–675. https://doi.org/10.1007/s10456-018-9621-x
Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ (2014) Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15(2):154–168. https://doi.org/10.1016/j.stem.2014.06.008
Picoli CC, Coimbra-Campos LMC, Guerra DAP, Silva WN, Prazeres PHDM, Costa AC, Magno LAV, Romano-Silva MA, Mintz A, Birbrair A (2019) Pericytes act as key players in spinal cord injury. Am J Pathol 189(7):1327–1337. https://doi.org/10.1016/j.ajpath.2019.03.008
Santos GSP, Magno LAV, Romano-Silva MA, Mintz A, Birbrair A (2019) Pericyte plasticity in the brain. Neurosci Bull 35(3):551–560. https://doi.org/10.1007/s12264-018-0296-5
Almeida VM, Paiva AE, Sena IFG, Mintz A, Magno LAV, Birbrair A (2018) Pericytes make spinal cord breathless after injury. Neuroscientis 24(5):440–447. https://doi.org/10.1177/1073858417731522
Dias Moura Prazeres PH, Sena IFG, Borges IDT, de Azevedo PO, Andreotti JP, de Paiva AE, de Almeida VM, de Paula Guerra DA, Pinheiro Dos Santos GS, Mintz A, Delbono O, Birbrair A (2017) Pericytes are heterogeneous in their origin within the same tissue. Dev Biol 427(1):6–11. https://doi.org/10.1016/j.ydbio.2017.05.001
Costa MA, Paiva AE, Andreotti JP, Cardoso MV, Cardoso CD, Mintz A, Birbrair A (2018) Pericytes constrict blood vessels after myocardial ischemia. J Mol Cell Cardiol 116:1–4. https://doi.org/10.1016/j.yjmcc.2018.01.014
Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O (2013) Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Res 10(1):67–84. https://doi.org/10.1016/j.scr.2012.09.003
Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD (2012) Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487(7408):443–448. https://doi.org/10.1038/nature11314
Collier RJ (2001) Understanding the mode of action of diphtheria toxin: a perspective on progress during the 20th century. Toxicon 39(11):1793–1803. https://doi.org/10.1016/s0041-0101(01)00165-9
Pappenheimer AM Jr, Harper AA, Moynihan M, Brockes JP (1982) Diphtheria toxin and related proteins: effect of route of injection on toxicity and the determination of cytotoxicity for various cultured cells. J Infect Dis 145(1):94–102. https://doi.org/10.1093/infdis/145.1.94
Yamaizumi M, Mekada E, Uchida T, Okada Y (1978) One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell 15(1):245–250. https://doi.org/10.1016/0092-8674(78)90099-5
Klages K, Mayer CT, Lahl K, Loddenkemper C, Teng MW, Ngiow SF, Smyth MJ, Hamann A, Huehn J, Sparwasser T (2010) Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res 70(20):7788–7799. https://doi.org/10.1158/0008-5472.CAN-10-1736
Lesokhin AM, Hohl TM, Kitano S, Cortez C, Hirschhorn-Cymerman D, Avogadri F, Rizzuto GA, Lazarus JJ, Pamer EG, Houghton AN, Merghoub T, Wolchok JD (2012) Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res 72(4):876–886. https://doi.org/10.1158/0008-5472.CAN-11-1792
Li X, Kostareli E, Suffner J, Garbi N, Hammerling GJ (2010) Efficient Treg depletion induces T-cell infiltration and rejection of large tumors. Eur J Immunol 40(12):3325–3335. https://doi.org/10.1002/eji.201041093
Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y (2006) CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol 176(3):1582–1587. https://doi.org/10.4049/jimmunol.176.3.1582
Teng MW, Ngiow SF, von Scheidt B, McLaughlin N, Sparwasser T, Smyth MJ (2010) Conditional regulatory T-cell depletion releases adaptive immunity preventing carcinogenesis and suppressing established tumor growth. Cancer Res 70(20):7800–7809. https://doi.org/10.1158/0008-5472.CAN-10-1681
Teng MW, Swann JB, von Scheidt B, Sharkey J, Zerafa N, McLaughlin N, Yamaguchi T, Sakaguchi S, Darcy PK, Smyth MJ (2010) Multiple antitumor mechanisms downstream of prophylactic regulatory T-cell depletion. Cancer Res 70(7):2665–2674. https://doi.org/10.1158/0008-5472.CAN-09-1574
Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF, Xu Y, Pogoriler E, Terzulli SL, Kuk D, Panageas KS, Ritter G, Sznol M, Halaban R, Jungbluth AA, Allison JP, Old LJ, Wolchok JD, Gnjatic S (2011) Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci USA 108(40):16723–16728. https://doi.org/10.1073/pnas.1110814108
Yuan J, Ginsberg B, Page D, Li Y, Rasalan T, Gallardo HF, Xu Y, Adams S, Bhardwaj N, Busam K, Old LJ, Allison JP, Jungbluth A, Wolchok JD (2011) CTLA-4 blockade increases antigen-specific CD8(+) T cells in prevaccinated patients with melanoma: three cases. Cancer Immunol Immunother 60(8):1137–1146. https://doi.org/10.1007/s00262-011-1011-9
Fridman WH, Pages F, Sautes-Fridman C, Galon J (2012) The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12(4):298–306. https://doi.org/10.1038/nrc3245
Rabinovich GA, Gabrilovich D, Sotomayor EM (2007) Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol 25:267–296. https://doi.org/10.1146/annurev.immunol.25.022106.141609
Tzekova N, Heinen A, Kury P (2014) Molecules involved in the crosstalk between immune- and peripheral nerve Schwann cells. J Clin Immunol 34(Suppl 1):S86-104. https://doi.org/10.1007/s10875-014-0015-6
Ydens E, Lornet G, Smits V, Goethals S, Timmerman V, Janssens S (2013) The neuroinflammatory role of Schwann cells in disease. Neurobiol Dis 55:95–103. https://doi.org/10.1016/j.nbd.2013.03.005
Goncalves NP, Vaegter CB, Andersen H, Ostergaard L, Calcutt NA, Jensen TS (2017) Schwann cell interactions with axons and microvessels in diabetic neuropathy. Nat Rev Neurol 13(3):135–147. https://doi.org/10.1038/nrneurol.2016.201
Neal JW, Gasque P (2016) The role of primary infection of Schwann cells in the aetiology of infective inflammatory neuropathies. J Infect 73(5):402–418. https://doi.org/10.1016/j.jinf.2016.08.006
Gabrilovich DI, Ciernik IF, Carbone DP (1996) Dendritic cells in antitumor immune responses. I. Defective antigen presentation in tumor-bearing hosts. Cell Immunol 170(1):101–110. https://doi.org/10.1006/cimm.1996.0139
Kim KD, Choi S-C, Kim A, Choe Y-K, Choe IS, Lim J-S (2001) Dendritic cell-tumor coculturing vaccine can induce antitumor immunity through both NK and CTL interaction. Int Immunopharmacol 1(12):2117–2129
Gabrilovich DI, Nadaf S, Corak J, Berzofsky JA, Carbone DP (1996) Dendritic cells in antitumor immune responses: II. Dendritic cells grown from bone marrow precursors, but not mature DC from tumor-bearing mice, are effective antigen carriers in the therapy of established tumors. Cell Immunol 170(1):111–119
Mrass P, Takano H, Ng LG, Daxini S, Lasaro MO, Iparraguirre A, Cavanagh LL, von Andrian UH, Ertl HC, Haydon PG, Weninger W (2006) Random migration precedes stable target cell interactions of tumor-infiltrating T cells. J Exp Med 203(12):2749–2761. https://doi.org/10.1084/jem.20060710
Alvarez-Dominguez C, Calderon-Gonzalez R, Teran-Navarro H, Salcines-Cuevas D, Garcia-Castano A, Freire J, Gomez-Roman J, Rivera F (2017) Dendritic cell therapy in melanoma. Ann Transl Med 5(19):386. https://doi.org/10.21037/atm.2017.06.13
Jacobs JF, Nierkens S, Figdor CG, de Vries IJ, Adema GJ (2012) Regulatory T cells in melanoma: the final hurdle towards effective immunotherapy? Lancet Oncol 13(1):e32-42. https://doi.org/10.1016/S1470-2045(11)70155-3
Sanmamed MF, Chen L (2018) A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell 175(2):313–326. https://doi.org/10.1016/j.cell.2018.09.035
Darvin P, Toor SM, Sasidharan Nair V, Elkord E (2018) Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med 50(12):1–11. https://doi.org/10.1038/s12276-018-0191-1
Hargadon KM, Johnson CE, Williams CJ (2018) Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol 62:29–39. https://doi.org/10.1016/j.intimp.2018.06.001
Blank CU, Haining WN, Held W, Hogan PG, Kallies A, Lugli E, Lynn RC, Philip M, Rao A, Restifo NP, Schietinger A, Schumacher TN, Schwartzberg PL, Sharpe AH, Speiser DE, Wherry EJ, Youngblood BA, Zehn D (2019) Defining “T cell exhaustion.” Nat Rev Immunol 19(11):665–674. https://doi.org/10.1038/s41577-019-0221-9
Wherry EJ, Kurachi M (2015) Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 15(8):486–499. https://doi.org/10.1038/nri3862
Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP (2008) Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood 112(2):362–373. https://doi.org/10.1182/blood-2007-11-120998
Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C (2009) T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 31(5):787–798. https://doi.org/10.1016/j.immuni.2009.09.014
Safari-Alighiarloo N, Taghizadeh M, Rezaei-Tavirani M, Goliaei B, Peyvandi AA (2014) Protein-protein interaction networks (PPI) and complex diseases. Gastroenterol Hepatol Bed Bench 7(1):17–31
Taylor IW, Wrana JL (2012) Protein interaction networks in medicine and disease. Proteomics 12(10):1706–1716. https://doi.org/10.1002/pmic.201100594
Chen B, Fan W, Liu J, Wu FX (2014) Identifying protein complexes and functional modules–from static PPI networks to dynamic PPI networks. Brief Bioinform 15(2):177–194. https://doi.org/10.1093/bib/bbt039
Haack H, Hynes RO (2001) Integrin receptors are required for cell survival and proliferation during development of the peripheral glial lineage. Dev Biol 233(1):38–55. https://doi.org/10.1006/dbio.2001.0213
Lefcort F, Venstrom K, McDonald JA, Reichardt LF (1992) Regulation of expression of fibronectin and its receptor, alpha 5 beta 1, during development and regeneration of peripheral nerve. Development 116(3):767–782. https://doi.org/10.1242/dev.116.3.767
Peters JH, Chen GE, Hynes RO (1996) Fibronectin isoform distribution in the mouse. II. Differential distribution of the alternatively spliced EIIIB, EIIIA, and V segments in the adult mouse. Cell Adhes Commun 4(2):127–148. https://doi.org/10.3109/15419069609010767
McAllister SS, Gifford AM, Greiner AL, Kelleher SP, Saelzler MP, Ince TA, Reinhardt F, Harris LN, Hylander BL, Repasky EA, Weinberg RA (2008) Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell 133(6):994–1005. https://doi.org/10.1016/j.cell.2008.04.045
Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D (2005) VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438(7069):820–827. https://doi.org/10.1038/nature04186
Johansson M, Denardo DG, Coussens LM (2008) Polarized immune responses differentially regulate cancer development. Immunol Rev 222:145–154. https://doi.org/10.1111/j.1600-065X.2008.00600.x
Gatenby RA, Gillies RJ (2008) A microenvironmental model of carcinogenesis. Nat Rev Cancer 8(1):56–61. https://doi.org/10.1038/nrc2255
Rodella U, Negro S, Scorzeto M, Bergamin E, Jalink K, Montecucco C, Yuki N, Rigoni M (2017) Schwann cells are activated by ATP released from neurons in an in vitro cellular model of Miller Fisher syndrome. Dis Model Mech 10(5):597–603. https://doi.org/10.1242/dmm.027870
Campana WM (2007) Schwann cells: activated peripheral glia and their role in neuropathic pain. Brain Behav Immun 21(5):522–527. https://doi.org/10.1016/j.bbi.2006.12.008
Yang DP, Zhang DP, Mak KS, Bonder DE, Pomeroy SL, Kim HA (2008) Schwann cell proliferation during Wallerian degeneration is not necessary for regeneration and remyelination of the peripheral nerves: axon-dependent removal of newly generated Schwann cells by apoptosis. Mol Cell Neurosci 38(1):80–88. https://doi.org/10.1016/j.mcn.2008.01.017
Kadioglu E, De Palma M (2015) Cancer metastasis: perivascular macrophages under watch. Cancer Discov 5(9):906–908. https://doi.org/10.1158/2159-8290.CD-15-0819
Harney AS, Arwert EN, Entenberg D, Wang Y, Guo P, Qian BZ, Oktay MH, Pollard JW, Jones JG, Condeelis JS (2015) Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage-derived VEGFA. Cancer Discov 5(9):932–943. https://doi.org/10.1158/2159-8290.CD-15-0012
Manberg A, Skene N, Sanders F, Trusohamn M, Remnestal J, Szczepinska A, Aksoylu IS, Lonnerberg P, Ebarasi L, Wouters S, Lehmann M, Olofsson J, von Gohren AI, Domaniku A, De Schaepdryver M, De Vocht J, Poesen K, Uhlen M, Anink J, Mijnsbergen C, Vergunst-Bosch H, Hubers A, Klappe U, Rodriguez-Vieitez E, Gilthorpe JD, Hedlund E, Harris RA, Aronica E, Van Damme P, Ludolph A, Veldink J, Ingre C, Nilsson P, Lewandowski SA (2021) Altered perivascular fibroblast activity precedes ALS disease onset. Nat Med 27(4):640–646. https://doi.org/10.1038/s41591-021-01295-9
Soderblom C, Luo X, Blumenthal E, Bray E, Lyapichev K, Ramos J, Krishnan V, Lai-Hsu C, Park KK, Tsoulfas P, Lee JK (2013) Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J Neurosci 33(34):13882–13887. https://doi.org/10.1523/JNEUROSCI.2524-13.2013
Crisan M, Corselli M, Chen WC, Peault B (2012) Perivascular cells for regenerative medicine. J Cell Mol Med. https://doi.org/10.1111/j.1582-4934.2012.01617.x
Wanjare M, Kusuma S, Gerecht S (2013) Perivascular cells in blood vessel regeneration. Biotechnol J 8(4):434–447. https://doi.org/10.1002/biot.201200199
Bhatia A, Kumar Y (2011) Cancer-immune equilibrium: questions unanswered. Cancer Microenviron 4(2):209–217. https://doi.org/10.1007/s12307-011-0065-8
Passarelli A, Mannavola F, Stucci LS, Tucci M, Silvestris F (2017) Immune system and melanoma biology: a balance between immunosurveillance and immune escape. Oncotarget 8(62):106132–106142. https://doi.org/10.18632/oncotarget.22190
Mittal D, Gubin MM, Schreiber RD, Smyth MJ (2014) New insights into cancer immunoediting and its three component phases–elimination, equilibrium and escape. Curr Opin Immunol 27:16–25. https://doi.org/10.1016/j.coi.2014.01.004
Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW (2011) The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 105(1):93–103. https://doi.org/10.1038/bjc.2011.189
Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC (2001) Regulation of cutaneous malignancy by gammadelta T cells. Science 294(5542):605–609. https://doi.org/10.1126/science.1063916
He W, Hao J, Dong S, Gao Y, Tao J, Chi H, Flavell R, O’Brien RL, Born WK, Craft J, Han J, Wang P, Zhao L, Wu J, Yin Z (2010) Naturally activated V gamma 4 gamma delta T cells play a protective role in tumor immunity through expression of eomesodermin. J Immunol 185(1):126–133. https://doi.org/10.4049/jimmunol.0903767
Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA et al (1988) Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med 319(25):1676–1680. https://doi.org/10.1056/NEJM198812223192527
Gerber AL, Munst A, Schlapbach C, Shafighi M, Kiermeir D, Husler R, Hunger RE (2014) High expression of FOXP3 in primary melanoma is associated with tumour progression. Br J Dermatol 170(1):103–109. https://doi.org/10.1111/bjd.12641
Huntington ND, Vosshenrich CA, Di Santo JP (2007) Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat Rev Immunol 7(9):703–714. https://doi.org/10.1038/nri2154
Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, Nakajima A, Hirohashi S (2007) FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res 13(3):902–911. https://doi.org/10.1158/1078-0432.CCR-06-2363
Sacerdote P, Franchi S, Trovato AE, Valsecchi AE, Panerai AE, Colleoni M (2008) Transient early expression of TNF-alpha in sciatic nerve and dorsal root ganglia in a mouse model of painful peripheral neuropathy. Neurosci Lett 436(2):210–213. https://doi.org/10.1016/j.neulet.2008.03.023
Martucci C, Trovato AE, Costa B, Borsani E, Franchi S, Magnaghi V, Panerai AE, Rodella LF, Valsecchi AE, Sacerdote P, Colleoni M (2008) The purinergic antagonist PPADS reduces pain related behaviours and interleukin-1 beta, interleukin-6, iNOS and nNOS overproduction in central and peripheral nervous system after peripheral neuropathy in mice. Pain 137(1):81–95. https://doi.org/10.1016/j.pain.2007.08.017
Franchi S, Valsecchi AE, Borsani E, Procacci P, Ferrari D, Zaffa C, Sartori P, Rodella LF, Vescovi A, Maione S, Rossi F, Sacerdote P, Colleoni M, Panerai AE (2012) Intravenous neural stem cells abolish nociceptive hypersensitivity and trigger nerve regeneration in experimental neuropathy. Pain 153(4):850–861. https://doi.org/10.1016/j.pain.2012.01.008
Wang ZH, Zeng XY, Han SP, Fan GX, Wang JY (2012) Interleukin-10 of red nucleus plays anti-allodynia effect in neuropathic pain rats with spared nerve injury. Neurochem Res 37(8):1811–1819. https://doi.org/10.1007/s11064-012-0795-0
Keswani SC, Buldanlioglu U, Fischer A, Reed N, Polley M, Liang H, Zhou C, Jack C, Leitz GJ, Hoke A (2004) A novel endogenous erythropoietin mediated pathway prevents axonal degeneration. Ann Neurol 56(6):815–826. https://doi.org/10.1002/ana.20285
Campana WM, Li X, Shubayev VI, Angert M, Cai K, Myers RR (2006) Erythropoietin reduces Schwann cell TNF-alpha, Wallerian degeneration and pain-related behaviors after peripheral nerve injury. Eur J Neurosci 23(3):617–626. https://doi.org/10.1111/j.1460-9568.2006.04606.x
Takahashi M, Kawaguchi M, Shimada K, Konishi N, Furuya H, Nakashima T (2004) Cyclooxygenase-2 expression in Schwann cells and macrophages in the sciatic nerve after single spinal nerve injury in rats. Neurosci Lett 363(3):203–206. https://doi.org/10.1016/j.neulet.2004.03.040
Toews AD, Barrett C, Morell P (1998) Monocyte chemoattractant protein 1 is responsible for macrophage recruitment following injury to sciatic nerve. J Neurosci Res 53(2):260–267. https://doi.org/10.1002/(SICI)1097-4547(19980715)53:2%3c260::AID-JNR15%3e3.0.CO;2-A
Yajima Y, Narita M, Usui A, Kaneko C, Miyatake M, Narita M, Yamaguchi T, Tamaki H, Wachi H, Seyama Y, Suzuki T (2005) Direct evidence for the involvement of brain-derived neurotrophic factor in the development of a neuropathic pain-like state in mice. J Neurochem 93(3):584–594. https://doi.org/10.1111/j.1471-4159.2005.03045.x
Su WF, Wu F, Jin ZH, Gu Y, Chen YT, Fei Y, Chen H, Wang YX, Xing LY, Zhao YY, Yuan Y, Tang X, Chen G (2019) Overexpression of P2X4 receptor in Schwann cells promotes motor and sensory functional recovery and remyelination via BDNF secretion after nerve injury. Glia 67(1):78–90. https://doi.org/10.1002/glia.23527
Roger E, Martel S, Bertrand-Chapel A, Depollier A, Chuvin N, Pommier RM, Yacoub K, Caligaris C, Cardot-Ruffino V, Chauvet V, Aires S, Mohkam K, Mabrut JY, Adham M, Fenouil T, Hervieu V, Broutier L, Castets M, Neuzillet C, Cassier PA, Tomasini R, Sentis S, Bartholin L (2019) Schwann cells support oncogenic potential of pancreatic cancer cells through TGFbeta signaling. Cell Death Dis 10(12):886. https://doi.org/10.1038/s41419-019-2116-x
Ferdoushi A, Li X, Griffin N, Faulkner S, Jamaluddin MFB, Gao F, Jiang CC, van Helden DF, Tanwar PS, Jobling P, Hondermarck H (2020) Schwann cell stimulation of pancreatic cancer cells: a proteomic analysis. Front Oncol 10:1601. https://doi.org/10.3389/fonc.2020.01601
Salvo E, Tu NH, Scheff NN, Dubeykovskaya ZA, Chavan SA, Aouizerat BE, Ye Y (2021) TNFalpha promotes oral cancer growth, pain, and Schwann cell activation. Sci Rep 11(1):1840. https://doi.org/10.1038/s41598-021-81500-4
Ivanova E, Corona C, Eleftheriou CG, Bianchimano P, Sagdullaev BT (2021) Retina-specific targeting of pericytes reveals structural diversity and enables control of capillary blood flow. J Comp Neurol 529(6):1121–1134. https://doi.org/10.1002/cne.25011
Acknowledgements
Alexander Birbrair is supported by a research productivity fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-PQ2), a grant from Instituto Serrapilheira/Serra-1708-15285, a Grant from Pró-reitoria de Pesquisa/Universidade Federal de Minas Gerais (PRPq/UFMG) (Edital 05/2016); a grant from Fundação de Amparo à Pesquisa do Estado de Minas Gerais—FAPEMIG (Chamada N°01/2021—Demanda Universal, APQ-01321-21); a grant from FAPEMIG [Rede Mineira de Pesquisa Translacional em Imunobiológicos e Biofármacos no Câncer (REMITRIBIC, RED-00031-21)]; a grant from FAPEMIG [Rede Mineira de Engenharia de Tecidos e Terapia Celular (REMETTEC, RED-00570-16)]; a grant from FAPEMIG [Rede De Pesquisa Em Doenças Infecciosas Humanas E Animais Do Estado De Minas Gerais (RED-00313-16)]; and a grant from MCTIC/CNPq Nº 28/2018 (Universal/Faixa A). Akiva Mintz is supported by the National Institute of Health (1R01CA179072-01A1) and by the American Cancer Society Mentored Research Scholar grant (124443-MRSG-13-121-01-CDD). Edroaldo Lummertz da Rocha is supported by the Coordination for the Improvement of Higher Education Personnel (CAPES), the National Council of State Funding Agencies (CONFAP), the Serrapilheira Institute and the Foundation for Support of Research and Innovation of Santa Catarina (FAPESC). Marcelo Falchetti is supported by a postdoctoral fellowship from the Brazilian National Council for Scientific and Technological Development (CNPq), Brazil. Remo C. Russo is supported by a research productivity fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-PQ2) and a Grant from FAPEMIG (Chamada N°01/2021 – Demanda Universal, APQ-02571-21). Pedro A F Galante was supported by a research productivity fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico, a grant from Instituto Serrapilheira, and a grant from São Paulo Research Foundation (FAPESP), grant 2012/24731-1. Gabriela D. A. Guardia was supported by São Paulo Research Foundation (FAPESP), grant 2017/19541-2, and a fellowship from Hospital Sirio-Libanês, Young Scientist initiative. Caroline C. Picoli and Alinne C. Costa are supported by doctoral fellowships from CAPES. Bryan O. P. Gonçalves is supported by a doctoral fellowship from FAPEMIG. Gabryella S.P. Santos is supported by a doctoral fellowship from CNPq. Beatriz G. S. Rocha and Walison N. Silva are supported by master fellowships from CAPES. Milla R. Almeida is supported by a scientific initiation fellowship from CNPq. Pedro A. C. Costa is supported by a postdoctoral fellowship (PNPD) from CAPES. The authors also thank CAPI (UFMG) for microscopical technical support and Laboratory of Flow Cytometry at the Instituto de Ciências Biológicas/UFMG (http://labs.icb.ufmg.br/citometria/)” for providing the equipment and technical support for experiments involving flow cytometry.
Author information
Authors and Affiliations
Contributions
AB conceived and supervised the study; BGSR, CCP, BOPG, WNS, ACC, MMM, PACC, GSPS, MRA, LMS, YS, MF, GDAG, PPGG, RCR, RRR, MCXP, JHA, VACA, AK, HIN, ELR, PAFG, AB analyzed the data and discussed the results; AB was responsible for funding; AM, PSF, AB wrote the original draft; all authors contributed to and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors indicate no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Beatriz G. S. Rocha, Caroline C. Picoli and Bryan O. P. Gonçalves are co-first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
10456_2022_9858_MOESM7_ESM.tif
Supplementary Figure 1. Nerves infiltrate within the tumor microenvironment. A. Adult wild-type mice were orthotopically injected with Tramp-C2 prostate cancer cells. The tumors were surgically removed 2 weeks later for analysis. B. Percentage of blood vessels with or without Peripherin+ nerve fibers attached to them in the Tramp-C2 prostate cancer microenvironment (n=3 mice) (81.25 ± 4.54 % of blood vessels were not associated to Peripherin+ nerve fibers; p < 0.0001; ES =15.7L). C and D. Representative images of sections from orthotopic Tramp-C2 tumors show nerves within the prostate tumor microenvironment. C. All panels show the same area for different channels: Peripherin (marker of peripheral nerve fibers), CD31 (marker of endothelial cells), DAPI, and all three merged. The area in the white box is magnified showing a nerve fiber attached to a blood vessel within the prostate tumor microenvironment. D. All panels show the same area for different channels: PGP9.5 (neuronal marker), CD31, DAPI, and merged. E. Percentage of blood vessels with or without PGP9.5+ nerve fibers attached to them in the Tramp-C2 prostate cancer microenvironment (n=3 mice) (79.7 ± 6.2 % of blood vessels were not associated to PGP9.5+ nerve fibers; p < 0.0001; ES =9.8L). F, G and H. Sympathetic nerves are present in the prostate tumor microenvironment. F. Intra-prostatic injection of TdTomato-labeled PC-3 human prostate cancer cells in nude mice, and tumor analysis after 3 weeks. G. Representative photomicrographs of a prostate tumor section 3 weeks after PC3 cells injection, showing blood vessels with and without attached TH+ nerve fibers. H. Percentage of blood vessels with or without attached TH+ nerve fibers in the PC3 prostate tumor microenvironment after 3 weeks (n=3 mice) (83.6 ± 4.2 % of blood vessels were not associated to TH+ nerve fibers; p < 0.0001; ES =5.4L). Data are mean ± SEM. TH, tyrosine hydroxylase, sympathetic neuron marker. Statistical analysis: unpaired Student's t-tests. ES: effect size; Llarge (≥ 1.2). ***p <0.001. Scale bars, 50µm (C and D) and 10 µm (G). (TIF 5864 kb)
10456_2022_9858_MOESM8_ESM.tif
Supplementary Figure 2. GFAP+ cells are present in the microenvironment of syngeneic C57BL6 mouse prostate tumors. A. Adult wild-type mice orthotopically injected with Tramp-C2 prostate cancer cells. B. Percentages of GFAP+ cells attached or not to blood vessels in the Tramp-C2 tumor after 2 weeks (n=3 mice) (93.7 ± 2.7 % of GFAP+ cells were associated to blood vessels, while 6.3 ± 2.7 % were not associated to blood vessels; p < 0.0001; ES =21.75L). C. Prostate Tramp-C2 tumor surgically removed after 2 weeks. D. Representative image of a section from orthotopic Tramp-C2 tumor shows blood vessel-associated GFAP+ cells in C57BL/6 mice. All panels show the same area for different channels (GFAP, CD31, and the two images merged with DAPI). E, F and G. GFAP+ cells are present within RM1 prostate tumor microenvironment associated with blood vessels. E. Intra-prostatic injection of RM1 prostate cancer cell line (from Ras+Myc transformed mouse prostate carcinoma) in wild-type mice, and tumor analysis after 2 weeks. F. Percentages of GFAP+ cells attached or not to blood vessels in the RM1 tumor after 2 weeks (n=3 mice) (91.0 ± 5.7 % of GFAP+ cells were associated to blood vessels, while 9.0 ± 5.6 % were not associated to blood vessels; p < 0.0001; ES =9.11L). G. Representative photomicrographs of a prostate tumor section 2 weeks after RM1 cells injection, showing blood vessels with GFAP+ cells attached to it. Statistical analysis: unpaired Student's t-tests. ES: effect size; Llarge (≥ 1.2). ***p <0.001. Data are mean ± SEM. Scale bars, 10µm. (TIF 3503 kb)
10456_2022_9858_MOESM9_ESM.tif
Supplementary Figure 3. Tumor-infiltrating perivascular glial cells differ from pericytes. A. Representative photomicrographs of prostate tumor sections 2 weeks after Tramp-C2 cells orthotopic injection into Nestin-GFP/NG2-DsRed mice showing GFAP+ cells (blue). B. Percentage of NG2-DsRed+ cells expressing GFAP in the Tramp-C2 prostate tumor after 2 weeks (n=5 mice) (23.60 ± 4.69 % of NG2-DsRed+ cells were positive for GFAP; p < 0.001; ES = 5.3L). C. Representative photomicrographs of melanoma tumor sections 2 weeks after B16F10 cancer cells transplantation into Plp1-CreER/TdTom mice showing perivascular Plp1CreER+/TdTomato+ cells (red) not expressing PDGFRβ (green). D. Percentage of Plp1CreER+/TdTomato+ cells not expressing PDGFRβ in the B16F10 tumor after 2 weeks (n=5 mice) (99.9 ± 0.10 % of Plp1CreER+/TdTomato+ cells were negative for PDGFRβ; p < 0.001; ES = 998.0L). Statistical analysis: unpaired Student's t-tests. ES: effect size; Llarge (≥ 1.2). ***p <0.001. Data are mean ± SEM. Scale bars, 10µm. (TIF 3434 kb)
10456_2022_9858_MOESM10_ESM.tif
Supplementary Figure 4. Ablation of Schwann cells increases the number of tumor-infiltrating dendritic cells. Dendritic cells from B16F10–inoculated mice were analyzed ex vivo in Plp1-CreER-/iDTR+ (n = 6 mice) and Plp1-CreER+/iDTR+ (n = 9 mice) mice. A. Absolute number of dendritic cells from the melanomas of B16F10–inoculated mice (Plp1-CreER-/iDTR+: 2.72x106 ± 7.40x105 cells per mg of tumor; Plp1-CreER+/iDTR+: 8.60x106 ± 2.21x106 cells per mg of tumor; p=0.039; ES = 1.2L). B. Absolute number of HLA-DR+ dendritic cells dendritic cells from the melanomas of B16F10–inoculated mice (Plp1-CreER-/iDTR+: 2.27x107 ± 2.87x106 cells per mg of tumor; Plp1-CreER+/iDTR+: 5.01x107± 9.40x106 cells per mg of tumor; p = 0.029; ES = 1.3L). Statistical analysis: unpaired Student's t-tests one-tailed. ES: effect size; Llarge (≥ 1.2). Plp1-CreER-/iDTR+ (n = 6 mice) and Plp1-CreER+/iDTR+ (n = 9 mice). * p <0.05. Data are mean ± SEM. (TIF 526 kb)
10456_2022_9858_MOESM11_ESM.tif
Supplementary Figure 5. Tumor-infiltrating Treg cells are reduced by Schwann cell ablation. Regulatory T cells from B16F10–inoculated mice were analyzed ex vivo in Plp1-CreER-/iDTR+ (n = 6 mice) and Plp1-CreER+/iDTR+ (n = 9 mice) mice. A. Absolute number of Treg cells from the melanomas of B16F10–inoculated mice (Plp1-CreER-/iDTR+: 4.25x106 ± 7.18x105 cells per mg of tumor; Plp1-CreER+/iDTR+: 1.48x106 ± 4.20x105 cells per mg of tumor, p=0.006; ES = 1.8L). Column charts show proportion of CTLA-4 (B), PD-1 (C) and CTLA-4/PD-1 co-expressing (D) Treg cells from tumors of B16F10–inoculated mice. B. CTLA-4+ Regulatory T cells (Plp1-CreER-/iDTR+: 14.35 ± 1.95%; Plp1-CreER+/iDTR+: 17.06 ± 2.03%; p = 0.363; ES = 0.5S). C. PD-1+ Regulatory T cells (Plp1-CreER-/iDTR+: 19.10 ± 3.72%; Plp1-CreER+/iDTR+: 9.24 ± 3.31%; p = 0.036; ES = 1.1M). D. and CTLA-4+/PD-1+ Regulatory T cells (Plp1-CreER-/iDTR+: 4.01 ± 0.88%; Plp1-CreER+/iDTR+: 2.67 ± 0.70%; p = 0.1305; ES = 0.7M). Statistical analysis: unpaired Student's t-tests or Mann-Whitney Rank Sum Test one-tail. ES: effect size; Ttrivial (< 0.2); Ssmall (0.2–0.6); Mmedium (0.6–1.2); Llarge (≥ 1.2). *p<0.05 and **p<0.01. Data are mean ± SEM. (TIF 553 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rocha, B.G.S., Picoli, C.C., Gonçalves, B.O.P. et al. Tissue-resident glial cells associate with tumoral vasculature and promote cancer progression. Angiogenesis 26, 129–166 (2023). https://doi.org/10.1007/s10456-022-09858-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-022-09858-1