Abstract
Background
Retention of biological treatment provides a marker of drug effectiveness and patient satisfaction. Retention of golimumab was high in clinical trial extensions and real-world studies up to 5 years in patients with immune-mediated rheumatic diseases.
Objective
To assess the probability of real-world long-term retention of treatment with golimumab up to 7 years after treatment initiation.
Methods
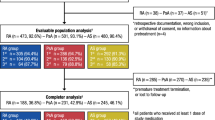
This retrospective noninterventional study involved analysis of the Spanish biological drugs registry, BIOBADASER. Adults who had ever received golimumab for rheumatoid arthritis (RA), axial spondyloarthritis (SpA), or psoriatic arthritis (PsA), and had initiated it > 6 months before the analysis date, were included.
Results
Among 685 patients (28.5% RA, 42.9% SpA, 28.6% PsA), the overall probability of retention of golimumab treatment since initiation was 71.7% (95% confidence interval 68.1–74.9) at year 1, 60.5% (56.5–64.2%) at year 2, 55.6% (51.5–59.5%) at year 3, 50.6% (46.2–54.8%) at year 4, 45.1% (40.1–50.0%) at year 5, 44.2% (39.0–49.3) at year 6, and 39.5% (32.8–46.2) at year 7. Retention was greater in patients with axial SpA or PsA versus RA (p < 0.001) and when golimumab was used as first-line treatment versus third or later lines (p < 0.001). Factors associated with greater golimumab retention in Cox regression included use as first-line biological therapy, having axial SpA or PsA rather than RA, and concomitant methotrexate therapy. Steroids were associated with lower retention.
Conclusion
In this real-world study of RA, axial SpA, and PsA patients, the retention rate of golimumab was 39.5% at year 7.
Key Points • Retention of biological treatment provides a marker of drug effectiveness and patient satisfaction. • This real-world study of 685 patients with rheumatoid arthritis (RA), axial spondyloarthritis (SpA), or psoriatic arthritis (PsA) showed that golimumab treatment had a retention rate up to 39.5% at year 7. • Greater golimumab retention was associated with use as first-line biological therapy, having axial SpA or PsA rather than RA, and concomitant methotrexate therapy |



Similar content being viewed by others
Data availability
Additional information and data of this study are available from the corresponding author upon reasonable request.
References
Tahir Z, Kavanaugh A (2018) The role of golimumab in inflammatory arthritis. A review of the evidence. Ther Adv Musculoskelet Dis 10(9):181–194
Kay J, Fleischmann R, Keystone E, Hsia EC, Hsu B, Zhou Y, Goldstein N, Braun J (2016) Five-year safety data from 5 clinical trials of subcutaneous golimumab in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol 43(12):2120–2130
Luttropp K, Dozier M, Justo N, Cornillie F, Kachroo S, Govoni M, Salomonsson S, Black CM, Khalifa A (2019) Real-world treatment persistence of golimumab in the management of immune-mediated rheumatic diseases in Europe: a systematic literature review. BMJ Open 9(5):e027456
Favalli EG, Pregnolato F, Biggioggero M, Becciolini A, Penatti AE, Marchesoni A, Meroni PL (2016) Twelve-year retention rate of first-line tumor necrosis factor inhibitors in rheumatoid arthritis: real-life data from a local registry. Arthritis Care Res (Hoboken) 68(4):432–439
Rotar Ž, Tomšič M, Praprotnik S, Slovenian Rheumatologists (2019) The persistence of golimumab compared to other tumour necrosis factor-α inhibitors in daily clinical practice for the treatment of rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis: observations from the Slovenian nation-wide longitudinal registry of patients treated with biologic disease-modifying antirheumatic drugs-BioRx.si. Clin Rheumatol 38(2):297–305
Deodhar A, Braun J, Inman RD, van der Heijde D, Zhou Y, Xu S, Han C, Hsu B (2015) Golimumab administered subcutaneously every 4 weeks in ankylosing spondylitis: 5-year results of the GO-RAISE study. Ann Rheum Dis 74(4):757–761
Emery P, Fleischmann RM, Strusberg I, Durez P, Nash P, Amante EJ, Churchill M, Park W, Pons-Estel B, Han C, Gathany TA, Xu S, Zhou Y, Leu JH, Hsia EC (2016) Efficacy and safety of subcutaneous golimumab in methotrexate-naive patients with rheumatoid arthritis: five-year results of a randomized clinical trial. Arthritis Care Res (Hoboken) 68(6):744–752
Keystone EC, Genovese MC, Hall S, Bae SC, Han C, Gathany TA, Xu S, Zhou Y, Leu JH, Hsia EC (2016) Safety and efficacy of subcutaneous golimumab in patients with active rheumatoid arthritis despite methotrexate therapy: final 5-year results of the GO-FORWARD trial. J Rheumatol 43(2):298–306
Hernandez MV, Sanchez-Piedra C, Garcia-Magallon B, Cuende E, Manero J, Campos-Fernandez C, Martin-Domenech R, Del Pino-Montes J, Manrique S, Castro-Villegas MC, Ruiz-Montesinos D, Sanchez-Alonso F, Diaz-Gonzalez F, Cea-Calvo L, Gómez-Reino JJ, BIOBADASER Study Group (2019) Factors associated with long-term retention of treatment with golimumab in a real-world setting: an analysis of the Spanish BIOBADASER registry. Rheumatol Int 39(3):509–515
Wolfe F, Michaud K, Dewitt EM (2004) Why results of clinical trials and observational studies of antitumour necrosis factor (anti-TNF) therapy differ: methodological and interpretive issues. Ann Rheum Dis 63(Suppl 2):ii13–ii17
Svedbom A, Storck C, Kachroo S, Govoni M, Khalifa A (2017) Persistence with golimumab in immune-mediated rheumatic diseases: a systematic review of real-world evidence in rheumatoid arthritis, axial spondyloarthritis, and psoriatic arthritis. Patient Prefer Adherence 11:719–729
Smolen JS, Kay J, Doyle M, Landewé R, Matteson EL, Gaylis N, Wollenhaupt J, Murphy FT, Xu S, Zhou Y, Hsia EC (2015) Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor α inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther 17(1):14
Mourão AF, Ribeiro C, Borges J, Gonçalves MJ, Bernardes M, Fernandes S, Dezerto R, Laires P, Machado P, Eusébio M, Santos MJ, Canhão H (2017) Real-life effectiveness of golimumab in biologic-naïve patients with rheumatoid arthritis - data from the Rheumatic Diseases Portuguese Register (Reuma.pt). Acta Reumatol Port 42:141–149
Manara M, Caporali R, Favalli EG, Grosso V, Atzeni F, Sarzi Puttini P, Gorla R, Bazzani C, Fusaro E, Pellerito R, Rocchetta PA, Sinigaglia L (2017) Two-year retention rate of golimumab in rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis: data from the LORHEN registry. Clin Exp Rheumatol 35(5):804–809
Iannone F, Santo L, Anelli MG, Bucci R, Semeraro A, Quarta L, D'Onofrio F, Marsico A, Carlino G, Casilli O, Cacciapaglia F, Zuccaro C, Falappone PC, Cantatore FP, Muratore M, Lapadula G (2017) Golimumab in real-life settings: 2 years drug survival and predictors of clinical outcomes in rheumatoid arthritis, spondyloarthritis, and psoriatic arthritis. Semin Arthritis Rheum 47(1):108–114
Favalli EG, Sinigaglia L, Becciolini A, Grosso V, Gorla R, Bazzani C, Atzeni F, Sarzi Puttini PC, Fusaro E, Pellerito R, Caporali R (2018) Two-year persistence of golimumab as second-line biologic agent in rheumatoid arthritis as compared to other subcutaneous tumor necrosis factor inhibitors: real-life data from the LORHEN registry. Int J Rheum Dis 21(2):422–430
Thomas K, Flouri I, Repa A, Fragiadaki K, Sfikakis PP, Koutsianas C, Kaltsonoudis E, Voulgari PV, Drosos AA, Petrikkou E, Sidiropoulos P, Vassilopoulos D (2018) High 3-year golimumab survival in patients with rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis: real world data from 328 patients. Clin Exp Rheumatol 36(2):254–262
Aaltonen KJ, Joensuu JT, Pirilä L, Kauppi M, Uutela T, Varjolahti-Lehtinen T, Yli-Kerttula T, Isomäki P, Nordström D, Sokka T (2017) Drug survival on tumour necrosis factor inhibitors in patients with rheumatoid arthritis in Finland. Scand J Rheumatol 46(5):359–363
Michelsen B, Sexton J, Wierød A, Bakland G, Rødevand E, Krøll F, Kvien TK (2020) Four-year follow-up of inflammatory arthropathy patients treated with golimumab: data from the observational multicentre NOR-DMARD study. Semin Arthritis Rheum 50(1):12–16
Greenberg JD, Reed G, Decktor D, Harrold L, Furst D, Gibofsky A, Dehoratius R, Kishimoto M, Kremer JM, CORRONA Investigators (2012) A comparative effectiveness study of adalimumab, etanercept and infliximab in biologically naive and switched rheumatoid arthritis patients: results from the US CORRONA registry. Ann Rheum Dis 71(7):1134–1142
Agarwal SK, Glass RJ, Shadick NA, Coblyn JS, Anderson RJ, Maher NE, Weinblatt ME, Solomon DH (2008) Predictors of discontinuation of tumor necrosis factor inhibitors in patients with rheumatoid arthritis. J Rheumatol 35(9):1737–1744
Mahlich J, Sruamsiri R (2016) Persistence with biologic agents for the treatment of rheumatoid arthritis in Japan. Patient Prefer Adherence 10:1509–1519
Fautrel B, Belhassen M, Hudry C, Woronoff-Lemsi MC, Levy-Bachelot L, Van Ganse E, Tubach F (2020) Predictive factors of tumour necrosis inhibitor treatment persistence for rheumatoid arthritis: an observational study in 8052 patients. Joint Bone Spine 87(3):267–269
Cañete JD, Naranjo A, Calvo J, Ordás C, Aragón B, Nocea G, Roset M, Fernández-Nebro A (2019) Biological treatment patterns in patients with inflammatory joint diseases. Retrospective study with 4 years follow-up. Reumatol Clin S1699-258X(18)30259-6
Bhoi P, Bessette L, Bell MJ, Tkaczyk C, Nantel F, Maslova K (2017) Adherence and dosing interval of subcutaneous antitumour necrosis factor biologics among patients with inflammatory arthritis: analysis from a Canadian administrative database. BMJ Open 7(9):e015872
Reginster JY, Rabenda V, Neuprez A (2006) Adherence, patient preference and dosing frequency: understanding the relationship. Bone 38(4 Suppl 1):S2–S6
Calvo-Alén J, Monteagudo I, Salvador G, Vázquez-Rodríguez TR, Tovar-Beltrán JV, Vela P, Maceiras F, Bustabad S, Román-Ivorra JA, Díaz-Miguel C, Rosas J, Raya E, Carmona L, Cea-Calvo L, Arteaga MJ, Fernández S, Marras C (2017) Non-adherence to subcutaneous biological medication in patients with rheumatoid arthritis: a multicentre, non-interventional study. Clin Exp Rheumatol 35(3):423–430
Tymms K, Littlejohn G, Griffiths H, de Jager J, Bird P, Joshua F, Nash P, Handel M, McManus H, Butcher BE, Youssef P (2018) Treatment patterns among patients with rheumatic disease (rheumatoid arthritis (RA), ankylosing spondylitis (AS), psoriatic arthritis (PsA) and undifferentiated arthritis (UnA)) treated with subcutaneous TNF inhibitors. Clin Rheumatol 37(6):1617–1623
Calip GS, Adimadhyam S, Xing S, Rincon JC, Lee WJ, Anguiano RH (2017) Medication adherence and persistence over time with self-administered TNF-alpha inhibitors among young adult, middle-aged, and older patients with rheumatologic conditions. Semin Arthritis Rheum 47(2):157–164
Tkacz J, Ellis L, Bolge SC, Meyer R, Brady BL, Ruetsch C (2014) Utilization and adherence patterns of subcutaneously administered anti-tumor necrosis factor treatment among rheumatoid arthritis patients. Clin Ther 36(5):737–747
Dalén J, Svedbom A, Black CM, Lyu R, Ding Q, Sajjan S, Sazonov V, Kachroo S (2016) Treatment persistence among patients with immune-mediated rheumatic disease newly treated with subcutaneous TNF-alpha inhibitors and costs associated with non-persistence. Rheumatol Int 36(7):987–995
Takacs P, Lathia U, Shin J, Nantel F (2019) Persistence to subcutaneous biological agents in Hungarian patients treated for inflammatory arthritis. Patient Prefer Adherence 13:157–163
Stober C, Ye W, Guruparan T, Htut E, Clunie G, Jadon D (2018) Prevalence and predictors of tumour necrosis factor inhibitor persistence in psoriatic arthritis. Rheumatology (Oxford) 57(1):158–163
Flouri ID, Markatseli TE, Boki KA, Papadopoulos I, Skopouli FN, Voulgari PV, Settas L, Zisopoulos D, Iliopoulos A, Geborek P, Drosos AA, Boumpas DT, Sidiropoulos P (2018) Comparative analysis and predictors of 10-year tumor necrosis factor inhibitors drug survival in patients with spondyloarthritis: first-year response predicts longterm drug persistence. J Rheumatol 45(6):785–794
Souto A, Maneiro JR, Gómez-Reino JJ (2016) Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology (Oxford) 55(3):523–534
Keystone EC, Genovese MC, Klareskog L, Hsia EC, Hall ST, Miranda PC, Pazdur J, Bae SC, Palmer W, Zrubek J, Wiekowski M, Visvanathan S, Wu Z, Rahman MU, GO-FORWARD Study (2009) Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study. Ann Rheum Dis 68(6):789–796
McCulley CB, Barton JL, Cannon GW, Sauer BC, Teng CC, George MD, Caplan L, England BR, Mikuls TR, Baker JF (2019) Body mass index and persistence of conventional DMARDs and TNF inhibitors in rheumatoid arthritis. Clin Exp Rheumatol 37(3):422–428
Singh S, Facciorusso A, Singh AG, Vande Casteele N, Zarrinpar A, Prokop LJ, Grunvald EL, Curtis JR, Sandborn WJ (2018) Obesity and response to anti-tumor necrosis factor-α agents in patients with select immune-mediated inflammatory diseases: a systematic review and meta-analysis. PLoS One 13(5):e0195123
Acknowledgements
The authors thank Kathy Croom and David P. Figgitt, Ph.D., ISMPP CMPP™, Content Ed Net, for providing medical writing/editorial assistance in the preparation of the manuscript, with funding from MSD Spain.
Funding
BIOBADASER is the Spanish registry of biological drugs, which is owned by the Spanish Agency of Medicines (https://www.aemps.gob.es/en/home.htm) and the Spanish Society of Rheumatology (https://www.ser.es/), and has additional financial support from different pharmaceutical companies. This specific analysis was funded by MSD Spain.
Author information
Authors and Affiliations
Contributions
M P-S, CS-P, FD-G, MJA, LC-C, and JJG-R were involved in the design of the study. Statistical analysis was independently executed by CS-P and FD-G, with overview from the abovementioned authors. LC-C and MJA, employees of MSD Spain, were involved in the design of the study but were not involved in data collection and had no access to the data source. The rest of the authors are regular investigators of BIOBADASER, were involved in data collection, and made substantial contributions to the current work. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
Manuel Pombo-Suarez: consulting fee from MSD; Blanca Garcia-Magallón: payments for consultancy, lectures, presentations, or travel expenses by MSD, Pfizer, Bristol-Myers, Amgen, Celgene, and Menarini; Sara Manrique-Arija: payments for consultancy, lectures, presentations, or travel expenses by Abbvie, Janssen, Sanofi, Novartis, Pfizer, Lilly, MSD, and Menarini; José Campos: payments for consultancy, lectures, presentations, or travel expenses by Pfizer, MSD, Novartis, Lilly, Roche, and Sanofi; Maria J. Arteaga: full-time employee at Medical Affairs, MSD, Spain; Luis Cea-Calvo: full-time employee at Medical Affairs, MSD, Spain; Federico Díaz-González: grant from MSD; Juan J. Gómez-Reino: payments for consultancy, lectures, and grants from MSD. The rest of the authors declared no conflict of interest.
Informed consent
All patients signed informed consent to be included in the BIOBADASER registry. Informed consent included consent for subsequent analysis, such as in the present analysis. Patients’ information was managed as anonymized aggregated data, and as approved by the Clinical Research Committee, specific informed consent for this analysis was not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 30 kb)
Rights and permissions
About this article
Cite this article
Pombo-Suarez, M., Sanchez-Piedra, C., Garcia-Magallón, B. et al. Factors associated with long-term retention of treatment with golimumab in rheumatoid arthritis, axial spondyloarthritis, and psoriatic arthritis: an analysis of the Spanish BIOBADASER registry. Clin Rheumatol 40, 3979–3988 (2021). https://doi.org/10.1007/s10067-021-05742-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-021-05742-3