Abstract
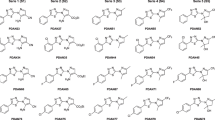
In this work, a novel series of ethyl and methyl quinoxaline-7-carboxylate 1,4-di-N-oxide derivatives were evaluated in vitro on Trypanosoma cruzi trypomastigotes and Leishmania mexicana promastigotes, and cytotoxicity activity in murine macrophages was tested. In silico molecular docking simulations of trypanothione reductase were also done. Three compounds of 33 quinoxaline-7-carboxylate 1,4-di-N-oxide derivatives showed better anti-T. cruzi activity than nifurtimox and beznidazole; two compounds had better anti-leishmanial activity that amphotericin-B, and two compounds showed better activity against both parasites than reference drugs. Compounds M2, M7, M8 and E5, showed low cytotoxic activity on the host cell. The in silico studies suggest that compound M2 is a potential trypanothione reductase inhibitor.



Similar content being viewed by others
References
Aguilera-Venegas B, Olea-Azar C, Norambuena E, Aran VJ, Mendizábal F, Lapier M, Maya JD, Kemmerling U, López-Muñoz R (2011) ESR, electrochemical, molecular modeling and biological evaluation of 4-substituted and 1,4-disubstituted 7-nitroquinoxalin-2-ones as potential anti-Trypanosoma cruzi agents. Spectrochim Acta A Mol Biomol Spectrosc 78:1004–1012
Aguirre G, Cerecetto H, Di Maio R, Gonzalez M, Alfaro ME, Jaso A, Zarranz B, Ortega MA, Aldana I, Monge-Vega A (2004) Quinoxaline N, N′-dioxide derivatives and related compounds as growth inhibitors of Trypanosoma cruzi. Structure–activity relationships. Bioorg Med Chem Lett 14:3835–3839
Baiocco P, Colotti G, Franceschini S, Ilari A (2009) Molecular basis of antimony treatment in Leishmaniasis. J Med Chem 52:2603–2612
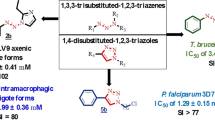
Barea C, Pabón A, Castillo D, Zimic M, Quiliano M, Galiano S, Perez-Silanes S, Monge A, Deharo E, Aldana I (2011) New salicylamide and sulfonamide derivatives of quinoxaline 1,4-di-N-oxide with antileishmanial and antimalarial activities. Bioorg Med Chem Lett 21:4498–4502
Benítez D, Cabrera M, Hernández P, Boiani L, Lavaggi ML, Di Maio R, Yaluff G, Serna E, Torres S, Ferreira ME, Vera de Bilbao N, Torres E, Perez-Silanes S, Solano B, Moreno E (2011) 3-Trifluoromethylquinoxaline N.N-dioxides as anti-trypanosomatid agents. Identification of optimal anti-T. cruzi agents and mechanism of action studies. J Med Chem 54:3624–3636
Bocanegra-García V, Villalobos-Rocha JC, Nogueda-Torres B, Lemus-Hernandez ME, Camargo-Ordoñez A, Rosas-García NM, Rivera G (2012) Synthesis and biological evaluation of new sulfonamide derivatives as potential anti-Trypanosoma cruzi agents. Med Chem 6:1039–1044
Bond CS, Zhang Y, Berriman M, Cunningham ML, Fairlamb AH, Hunter WN (1999) Crystal structure of Trypanosoma cruzi trypanothione reductase in complex with trypanothione, and the structure-based discovery of new natural product inhibitors. Structures 7:81
Brener Z (1962) Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev Inst Med Trop Sao Paulo 4:389–396
Cerecetto H, González M (2002) Chemotherapy of Chagas' disease: status and new developments. Curr Top Med Chem 2:1187–1213
Cerecetto H, Gonzalez M (2008) Anti-T. cruzi agents: our experience in the evaluation of more than five hundred compounds. Mini Rev Med Chem 8:1355–1383
Chung MC, Ferreira EI, Santos JL, Giarolla J, Rando DG, Almeida AE, Bosquesi PL, Menegon RF, Blau L (2007) Pro-drugs for the treatment of neglected diseases. Molecules 13:616–677
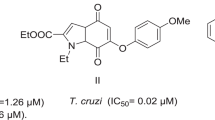
D’ocampo R, Moreno SNJ (1984) Quinoxaline-7-carboxylate 1,4-di-N-oxide as anti-trypanoosomatid agents. In: Pryor WA (ed) Free radicals in biology. Academic, New York, pp 243–288
De Souza W (2002) Basic cell biology of Trypanosoma cruzi. Curr Pharm Des 8:269–285
Dey A, Singh S (2006) Transfusion transmitted leishmaniasis: a case report and review of literature. Indian J Med Microbiol 24:165–170
Díaz-Chiguer D, Márquez-Navarro A, Nogueda-Torres B, León-Ávila G, Pérez-Villanueva J, Hernández-Campos A, Castillo R, Ambrosio J, Nieto-Meneses R, Yépez-Mulia L, Hernández-Luis F (2012) In vitro and in vivo trypanocidal activity of some benzimidazole derivatives against two strains of Trypanosoma cruzi. Acta Trop 122(1):108–112
Duque-Montano BE, Gómez-Caro LC, Sánchez-Sánchez M, Monge A, Hernández-Baltazar E, Rivera G, Torres-Angeles O (2013) Synthesis and in vitro evaluation of new ethyl and methyl quinoxaline-7- carboxylate 1,4-di-N-oxide against Entamoeba histolytica. Bioorg Med Chem 21:4550–4558
Estevez Y, Quiliano M, Burguete A, Cabanillas B, Zimic M, Málaga E, Verástegui M, Perez-Silanes S, Aldana I, Monge A, Castillo D, Deharo E (2011) Trypanocidal properties, structure-activity relationship and computational studies of quinoxaline 1,4-di-N-oxide derivatives. Exp Parasitol 127:745–751
Evans BE, Rittle KE, Bock MG, DiPardo RM, Freidinger RM, Whitter WL, Lundell GF, Veber DF, Anderson PS (1988) Methods for drug discovery: development of potent, selective, orally effective cholecystokinin antagonists? Med Chem 31:2235–2246
Gómez-Caro LC, Sánchez-Sánchez M, Bocanegra-García V, Rivera G, Monge A (2011) Synthesis of quinoxaline 1,4-di-N-oxide derivatives on solid support using room temperature and microwave-assisted solvent-free procedures. Quim Nova 34:1147–1151
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR (2012) Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminformat 4:17
Hetenyi C, van der Spoel D (2002) Efficient docking of peptides to proteins without prior knowledge of the binding site. Protein Sci 11:1729–1737
Leeson PD, Davis AM, Steele J (2004) Drug-like properties: guiding principles for design or chemical prejudice? Drug Discov Today 1:189–195
Lima LM, Barreiro EJ (2005) Bioisosterism: a useful strategy for molecular modification and drug design. Curr Med Chem 12:23–49
Melo F, Feytmans E (1998) Assessing protein structures with a non-local atomic interaction energy. J Mol Biol 277:1141–1152
Moncayo A, Ortiz-Yanine MI (2006) An update on Chagas’ disease (human American Tripanosomiasis). Ann Trop Med Parasitol 100:663–677
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) Autodock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 16:5–91
Murray HW, Berman JD, Davies CR, Saravia NG (2005) Advances in Leishmaniasis. Lancet 366:1561–1577
Paulino M, Iribarne F, Dubin M, Aguilera-Morales S, Tapia O, Stoppani AO (2005) The chemotherapy of Chagas’ disease: an overview. Mini Rev Med Chem 5:499–519
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF chimera visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612
Raaflaub J, Ziegler W (1979) Single-dose pharmacokinetics of the trypanosomicide benznidazole in man. Drug Res 29:1611–1614
Rivera N, Ponce YM, Arán VJ, Martínez C, Malagón F (2013) Biological assay of a novel quinoxalinone with antimalarial efficacy on Plasmodium yoelii yoelii. Parasitol Res 112(4):1523–1527
Romanha AJ, Castro SL, Soeiro Mde N, Lannes-Vieira J, Ribeiro I, Talvani A, Bourdin B, Blum B, Olivieri B, Zani C, Spadafora C, Chiari E, Chatelain E, Chaves G, Calzada JE (2010) In vitro and in vivo experimental models for drug screening and development for Chagas disease. Mem Inst Oswaldo Cruz 105:233–238
Sali A, Blundell TL (1993) Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234:779–815
Sanner MF (1999) Python: a programming language for software integration and development. J Mol Graph Model 17:57–61
Sephra N (2012) Multiple applications of alamar blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 12:12347–12360
Shimony O, Jaffe C (2008) Rapid fluorescent assay for screening drugs on Leishmania amastigotes. J Microbiol Methods 75:196–200
Singh BK, Sarkar N, Jagannadham MV, Dubey VK (2008) Modeled structure of trypanothione reductase of Leishmania infantum. India Bio Info Bank 6:444–447
Stoppani AOM (1999) Quimioterapia de la enfermedad de Chagas. Academia Nacional de Medicina Simposio Internacional Problemática de la enfermedad de Chagas. Facultad de Medicina Universidad de Buenos Aires Argentina 59(Supl. II):147–165
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem 31(2):455–461
World Health Organization. Chagas Disease: TDR. 2007, http://who.int/tdr/diseases/chagas/
Acknowledgments
Juan Carlos Villalobos-Rocha is the recipient of a scholarship (228946) from CONACyT, Mexico. Benjamin Nogueda-Torres, Luvia Sánchez-Torres, Virgilio Bocanegra-García, and Gildardo Rivera hold a scholarship from the Comisión de Operación y Fomento de Actividades Académicas (COFAA-Instituto Politécnico Nacional) and the Programa de Estímulos al Desempeño de los Investigadores (EDI-Instituto Politécnico Nacional). Our gratitude to Lilia C. Gómez-Caro, Mario Sánchez-Sánchez, and Jesus Villegas-Mendoza for their support in this project. We wish to express our gratitude to the CONACyT (CB-2010-01, clave 0157358) and the Secretaria de Investigación y Posgrado of Instituto Politecnico Nacional (SIP-20130637 and SIP-20130768) for their financial support of this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 62 kb)
Rights and permissions
About this article
Cite this article
Villalobos-Rocha, J.C., Sánchez-Torres, L., Nogueda-Torres, B. et al. Anti-Trypanosoma cruzi and anti-leishmanial activity by quinoxaline-7-carboxylate 1,4-di-N-oxide derivatives. Parasitol Res 113, 2027–2035 (2014). https://doi.org/10.1007/s00436-014-3850-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-3850-8