Abstract
Childhood abuse was inconsistently related to whole-brain cortical thickness in former studies. However, both childhood abuse and cortical thickness have been associated with depressive symptoms. We hypothesised that childhood abuse moderates the association between depressive symptoms and cortical thickness. In 1551 individuals of the general population, associations between whole-brain cortical thickness and the interaction of childhood abuse (emotional, physical, and sexual) and depressive symptoms were analysed using an ANCOVA. Linear regression analyses were used to estimate the same effect on the cortical thickness of 34 separate regions (Desikan-Killiany-atlas). A significant interaction effect of childhood abuse and depressive symptoms was observed for whole-brain cortical thickness (F(2, 1534) = 5.28, p = 0.007). A thinner cortex was associated with depressive symptoms in abused (t value = 2.78, p = 0.025) but not in non-abused participants (t value = − 1.50, p = 0.224). Focussing on non-depressed participants, a thicker whole-brain cortex was found in abused compared to non-abused participants (t value = − 2.79, p = 0.025). Similar interaction effects were observed in 12 out of 34 cortical regions. Our results suggest that childhood abuse is associated with reduced cortical thickness in subjects with depressive symptoms. In abused subjects without depressive symptoms, larger cortical thickness might act compensatory and thus reflect resilience against depressive symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The American Professional Society on the Abuse of Children (APSAC) defines childhood abuse as “words or overt actions that cause harm, potential harm, or threat of harm to a child” [1] and differentiates between emotional, physical, and sexual abuse. In Germany, the prevalence of emotional abuse was estimated at 6.5%, physical abuse at 6.7%, and 7.6% at sexual abuse [2].
Childhood abuse initiates physiological and psychological stress-response systems [3], contributing to long-term changes in epigenetic processes, gene regulation, and brain development [4, 5]. Thus, childhood abuse is associated with higher lifetime risks of somatic illnesses such as metabolic syndrome, cardiovascular and respiratory disorders [6]. Additionally, childhood abuse is associated with the development of mental diseases such as major depressive disorder (MDD) [7, 8] and posttraumatic stress disorder [8]. Therefore, the genesis of mental diseases is, besides genetic factors, influenced by environmental aspects such as childhood abuse, probably through epigenetic mechanisms [9]. Hence, structural alterations may precede the onset of the mental disease [9]. In contrast, Teicher et al. [5] stated in their review in Nature Reviews Neuroscience that certain alterations in maltreated subjects are not linked to psychopathology and that maltreated subjects could also differ in brain changes which help them compensate [5]. Thus, understanding how childhood abuse increases the risk of physical and mental diseases and, thus, enabling the implementation of focused prevention programs to reduce pathological consequences is of high demand [5]. The exact mechanisms behind these associations are still elusive.
Several studies suggest that different patterns of brain alterations are associated with different types of maltreatment. Thus, parental verbal abuse was linked to a reduction in the auditory cortex [10] and sexual abuse was associated with a reduced extended genital representation in the sensomotoric cortex [11]. On the other hand, social deprivation was associated with reductions in regions of the association cortex processing social stimuli [12]. To address these findings, we focussed only on the consequences of childhood abuse on the cortex.
More recently, Tozzi et al. [13] analysed interaction effects of severity and type of childhood maltreatment (abuse or neglect) and MDD on cortical thickness in 3,872 participants within the ENIGMA consortium [13]. They found associations of childhood maltreatment severity with reductions of thinner cortices in the banks of the superior temporal sulcus and supramarginal gyrus [13]. However, they did neither find an interaction effect between childhood maltreatment and MDD, nor a main effect of MDD on cortical thickness. Within the ENIGMA consortium, Schmaal et al. [14] analysed the cortical thickness in 1,902 MDD patients and 7658 controls. They found thinner cortices in MDD patients in the temporal and frontal lobes, including the orbitofrontal cortex [14]. Further, Lim et al. [15] analysed a youth sample (excluding sexual abuse) and found lower cortical thickness in abused participants with psychiatric comorbidities in comparison to healthy non-abused participants. Given that childhood abuse is a crucial risk factor for the development of MDD, it is challenging to disentangle the contribution of both conditions to the cortical thickness [15]. Former findings show divergent results on how depression and childhood abuse affect the cortex. Investigating if and how the interaction of depression and childhood abuse might explain some of these differential outcomes, is a relevant concern.
Although the ENIGMA studies investigating the childhood-maltreatment-MDD-interaction on cortical regions analysed large sample sizes [13], there are limitations essential to consider: covariates such as level of education, alcohol consumption, smoking behaviour, and obesity were missing. However, as these parameters are frequently associated with childhood abuse, MDD, and cortical thickness, they could act as confounders [16, 17]. Further, the inclusion of many different samples increased the heterogeneity of the overall sample which might have lowered the effect sizes [18].
To address these limitations, we used a sample of 1,551 participants from the population-based Study of Health in Pomerania (SHIP) to analyse the interaction of childhood abuse and depressive symptoms on cortical thickness. We focused on current depressive symptoms, which were associated with childhood maltreatment in former studies [19, 20]. More precisely, this study regressed whole-brain cortical thickness in addition to the cortical thickness of 34 single cortical regions on the interaction effect between childhood abuse (yes/no) and depressive symptoms (none/mild/moderate to severe). Previous research revealed differential results on how depression and childhood abuse affect the cortex. We hypothesise that the interaction of childhood abuse and depressive symptoms is associated with the cortical thickness and might explain some of the diverse results.
Methods
Sample
SHIP is a population-based cohort study [21]. Local registries randomly drew the study sample of West Pomerania between 2008 and 2011 (SHIP-Trend-0: N = 4420). For more details see Völzke et al. [21]. SHIP-Trend-0 participants free of exclusion criteria (e.g. cardiac pacemakers, pregnancy) were invited for a whole-body magnetic resonance imaging (MRI) [22]. MRI of the head was available for 2,154 participants of SHIP-Trend-0 [23]. MRI scans of sufficient quality were available for 1,986 participants (see subdivision Magnetic Resonance Imaging for the detailed selection process). This study design excluded participants from the following analyses if the information on depressive symptoms (n = 60) or childhood abuse (n = 138) was missing or due to incomplete covariate data (n = 22; see subdivision Statistical Analyses). Finally, as we aimed to compare participants without any history of abuse or neglect with abused participants, we excluded participants if they reported an experience of neglect, but not abuse (n = 215). Thus, our final sample comprised 1,551 participants.
The institutional review board of the University Greifswald approved SHIP. Examinations and assessments have been conducted by the declaration of Helsinki, including written informed consent.
Measures
Interview
Sociodemographic data including age, sex, education level, smoking behaviour, intake of antidepressants (yes/no) and alcohol consumption was collected via a computer-assisted face-to-face interview. According to the German school system, educational level was divided into < 10 years (low), = 10 years (medium), and > 10 years of school education (high). SHIP-Trend-0 categorised smoking behaviour into never, ex-, and current smokers. Alcohol consumption was defined as the mean intake of gram ethanol per day over the past 30 days according to Baumeister et al. [24]. Afterwards, participants underwent a physical examination including the measurement of the waist circumference, weight, and height. The examiner measured the waist circumference with the participant standing on both feet to the nearest 0.1 cm. Weight was measured in light clothes to the nearest 0.1 kg. Height was measured to the nearest 1 cm. Participants were asked to report the medication used during the past 7 days and to bring their package containers. Medication was categorised according to the ATC-Index [25]. Antidepressants were defined as ATC codes N06A.
Childhood abuse
To enquire about the exposure to childhood abuse, this study used the German version of the Childhood-Trauma-Questionnaire (CTQ) [26, 27]. The CTQ is a 28-item questionnaire assessing maltreatment in childhood and youth [27, 28]. The CTQ measures different exposures of maltreatment in five subscales: emotional, sexual, and physical abuse, emotional and physical neglect. Participants rated each item on a 5-point Likert scale, from “not experienced” to “very often experienced”. The CTQ is an established instrument with decent reported validity (depression: r = 0.36, anxiety: r = 0.40, health: r = − 0.23) and reliability (emotional abuse: Cronbach’s α = 0.87, physical abuse: α = 0.80, sexual abuse: α = 0.89) with a Cronbach’s α of 0.94 for the whole questionnaire. For an overview of the German version see Klinitzke et al. [29]. Note again that, as we aimed to compare participants free of moderate to severe childhood maltreatment with abused participants, we excluded participants who exclusively reported emotional and/or physical neglect (emotional neglect ≥ 15; physical neglect ≥ 10) from the study (see section Sample). This exclusion criterion, however, did not take effect if participants reported co-experience of abuse and neglect. A dichotomized variable of childhood abuse was generated including sexual, physical, and emotional abuse. This study categorised participants to the abused group if they reached the defined cut-off score for moderate abuse in at least one of the three subscales (sexual abuse ≥ 8; emotional abuse ≥ 13; physical abuse ≥ 10) [27]. Participants were categorised to the non-abused group when they did neither score on the abuse nor the neglect subscales.
Depressive symptoms
To enquire depressive symptoms, this study used the Patient-Health-Questionnaire-9 (PHQ-9) [30]. The PHQ-9 assesses the existence of depressive symptoms with nine items, based on the nine criteria for MDD of the Diagnostic and Statistical Manual of Mental Disorders 4th Revision (DSM-IV). A 4-point Likert scale assesses the symptoms from having “no symptoms” to “symptoms every day”. The PHQ-9 has a suitable internal consistency with a Cronbach’s α of 0.88 [31]. Based on the summary score of the PHQ-9, this study design assorted participants into three groups according to the criteria of Kroenke et al. (2002): no depressive (≤ 4), mild depressive (5–9), and moderate to severe depressive symptoms (≥ 10).
Magnetic resonance imaging (MRI)
MRI of the head was available for 2154 participants of SHIP-Trend-0 [23]. A Siemens Magnetom Avanto scanner acquired T1-weighted images with following parameters: field strength = 1.5 T, orientation = axial plane, repetition time = 1900 ms, echo time = 3.37 ms, flip angle = 15°, slice thickness = 1 mm, and resolution 1 mm × 1 mm. Three scans were eliminated due to poor quality (strong frontal darkening). In addition, 100 scans were excluded due to structural abnormalities (e.g., tumours, cysts) and cerebral stroke. The image segmentation pipeline failed to process four scans. We excluded further 61 participants which were categorised as outliers in various cortical thickness regions (see below), resulting in an imaging sample of 1986 participants.
Parcellation of the cerebral cortex
The FreeSurfer image analysis suite (version 5.3) performed the cortical reconstruction and volumetric segmentation. This application suite is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu).
Briefly, this process included the removal of non-brain tissue using a hybrid watershed/surface deformation procedure [32], automated Talairach transformation, intensity normalization [33], tessellation of the grey/white matter boundary, and automated topology correction [34, 35]. Further, surface deformation follows intensity gradients to optimally place the grey/white and grey/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class [36,37,38].
Individual cortical models were registered to a spherical atlas [39] and parcelled into 68 units with respect to gyral and sulcal structure [40, 41]. Average thickness of each region was computed. Statistical quality control was performed by excluding cases with thickness values higher/lower than four standard deviations from the whole sample mean after adjustment for age, sex, body height, and the interaction of age with sex. 61 participants were excluded based on this criterion.
Cortical thicknesses were averaged across both hemispheres resulting in 34 brain region parameters. Besides, whole-brain cortical thickness was defined as the average of the thicknesses of the left and right hemisphere. The estimated intracranial volume (ICV) was generated by FreeSurfer.
Statistical analysis
All analyses were adjusted for sex, age, age2, sex-age-interaction, estimated intracranial volume (ICV), educational level, alcohol consumption, smoking behaviour, waist-to-height ratio, and body mass index (BMI).
To analyse the main and interaction effects of childhood abuse (yes/no) and depressive symptoms (none/mild/moderate to severe) on whole-brain cortical thickness, we implemented three models for an analysis of variance with covariates (ANCOVA) with whole-brain cortical thickness as the dependent variable. Two models were implemented with the confounding variables and either childhood abuse or depressive symptoms as independent variable. The third model included the interaction term childhood abuse*depression. The same analysis model was conducted with depressive symptoms as a continuous variable in a linear regression by using the sum score of the PHQ-9 (see SI). In an additional model, we included the intake of antidepressants (yes/no) as a covariate in addition to the other covariates mentioned above (see SI). Levene’s Test of Equality estimated the variance homogeneity. The overall test on whole-brain cortical thickness tested with a significance level of p < 0.05. Afterwards, 9 post-hoc tests were calculated for the interaction groups using the estimated marginal means. Therefore, we computed pairwise comparisons between the abuse groups at each level of depression and vice versa. These tests were adjusted by sex, age, age2, sex-age-interaction (sex × age), BMI, waist-to-height ratio, alcohol consumption, educational level, smoking, and ICV. Educational level and smoking were dummy coded and, thus, represented by two dummy variables, respectively. We used the false discovery rate (FDR) method of Benjamini and Hochberg [42] to adjust the p values for multiple testing for the 9 post-hoc pairwise comparisons.
To investigate which brain regions are affected by the interaction of childhood abuse and depressive symptoms, the cortical thickness of 34 segmented cortical regions was examined in separate linear regression analyses. We implemented the childhood abuse*depression interaction term as the independent variable and each cortical region separately as the dependent variable. These tests were adjusted by sex, age, age2, sex-age-interaction, BMI, waist-to-height ratio, alcohol consumption, educational level (dummy coded), smoking (dummy coded), and ICV. To correct for multiple testing, FDR correction was used for 34 linear regression analyses.
Descriptive statistics revealed differences in sex proportions and depression level (Table 1) between the abuse and non-abuse group. Thus, abused participants (n = 120) were matched 1:1 with non-abused participants (n = 120) by sex (male, female), depressive symptoms (using the categories: non, mild, or moderate to severe), and age. We matched for age to adjust for possible age-education-interactions. Nearest neighbour matching was accomplished with the package MatchIt. Descriptive statistics of the matched sample are presented in Table SI2. We recalculated all analyses with the matched sample as sensitivity analyses.
A p value of < 0.05 was considered statistically significant. All statistical analysis were performed using R version 3.6.2 [43].
Results
Demographics
Descriptive statistics are summarised in Table 1. Overall, 1,429 participants reported no exposure to any childhood maltreatment, 122 (8.5%) experienced at least one type of childhood abuse. In the whole sample, 11.0% of the female population reported childhood abuse, thus women were significantly more often affected by childhood abuse than men (4.6%; χ2 = 18.77, p < 0.001). Further, 1,064 participants (68.6%) reported no current depressive symptoms, whereas 397 participants (25.6%) reported mild, and 90 participants (5.8%) reported moderate to severe symptom severity. The participants of the group allocation (abuse: yes/no) did not vary regarding age, waist circumference, smoking, BMI, and educational level. However, there were substantial variations across the two groups in the distribution of sexes (χ2 = 18.77, p < 0.001), ICV (F(2, 1548) = 5.47, p = 0.019), alcohol consumption (F(2, 1548) = 4.41, p = 0.036), and in the frequency of the depressive categories (χ2 = 55.21, p < 0.001). Descriptive characteristics of the study sample ordered by the severity of depressive symptoms are displayed in Table SI1.
Thus, to consider these differences in the analyses more clearly, a subsample matched for sex, depression level, and age was extracted. Table SI2 summarizes the descriptive statistics for the matched sample in the supplementary information. In the matched sample, the two abuse-groups did no longer vary in the distribution of sexes, ICV, and the depression level (non, mild, moderate to severe) validating the matching process.
Effects of childhood abuse and depressive symptoms on whole-brain cortical thickness
The Levene’s-Test was non-significant for the ANCOVA analysis indicating no violation of variance homogeneity. Analysing the influence of childhood abuse on whole-brain cortical thickness, we found no significant effects (F(1, 1534) = 1.64; p = 0.201). Further, no significant effects were observed for depressive symptoms (F(1, 1534) = 0.08; p = 0.925). However, there was a statistically significant two-way interaction between childhood abuse and current depressive symptoms on whole-brain cortical thickness (F(2, 1534) = 5.28, p = 0.007). In addition, there was also a statistically significant two-way interaction between childhood abuse and the continuous variable for depressive symptoms on whole-brain cortical thickness (F(2, 1534) = − 2.97, p = 0.003).
To investigate this interaction effect in more detail, post-hoc pairwise comparisons were calculated using the estimated marginal means (see Fig. 1 and Table SI3). We adjusted all nine post-hoc comparisons with the false discovery rate method of Benjamini and Hochberg.
Line plot showing pairwise comparisons between groups using the estimated marginal means and standard errors of whole-brain cortical thickness across all levels of childhood abuse and depression. In the abused-group (black line), participants with moderate to severe depressive symptoms had significantly thinner whole-brain cortices than non-depressed subjects (p = 0.025). In the non-depressed (left), whole-brain cortical thickness was larger in abused compared to non-abused participants (p = 0.025). See Table SI3 for further information
Pairwise comparisons were computed between the abuse groups (yes/no) at each level of depression (non, mild, and moderate to severe) and vice versa. In abused participants, we found a statistically significant difference between non-depressed participants and participants with moderate to severe depressive symptoms (t value = 2.78, pFDR = 0.025). However, there was no significant difference between non-depressed and mildly depressed participants (t value = 1.68, pFDR = 0.209), nor between mildly depressed and moderately to severe depressed participants (t value = 1.36, pFDR = 0.224). In contrast, in non-abused participants, no significant differences reached the level of significance across the factor of depression.
In non-depressed participants, we found significant differences between abused and non-abused participants (t-value = − 2.79, pFDR = 0.025). In contrast, childhood abuse had no significant effect in mildly depressed (t value = − 0.25, pFDR = 0.904), nor moderate to severely depressed participants (t value = 2.052, pFDR = 0.121).
Using the matched sample (N = 240), we recalculated all results. The two-way interaction between childhood abuse and depressive symptoms remained significant (F(2, 223) = 4.40, pFDR = 0.013). Post-hoc pairwise comparisons revealed statistically significant differences in the abuse group between non-depressed participants and participants with moderate to severe depressive symptoms (t value = 3.34; pFDR = 0.009) (Fig. SI1).
Effects of childhood abuse and depressive symptoms on regional cortical thickness
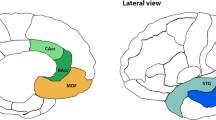
To investigate region-specific effects, we tested the interaction of childhood abuse and depressive symptoms on the cortical thicknesses of 34 cortical regions, separately. Table 2 and Fig. 2 present the results.
Associations between cortical thickness in 34 cortical regions and the interaction of depressive symptoms and childhood abuse. Colours represent exclusively significant t-values from linear regressions with the brain regions as the dependent variable and adjusted for sex, age, age-sex-interaction, age2, ICV, education, alcohol consumption, smoking, BMI, and waist-to-height ratio
After the FDR-correction for 34 cortical regions, the interaction effect was significantly associated with cortical thickness in 12 cortical regions within the inferior frontalis gyrus (pars opercularis, pars orbitalis, pars triangularis), supramarginal gyrus, inferior temporal gyrus, lateral occipital gyrus, lateral orbitofrontal cortex, pericalcarine cortex, precuneus, superior parietal lobule, superior temporal gyrus, and temporal pole (p < 0.05 after FDR correction). These cortical regions replicated the interaction effect as seen in the analysis of the whole-brain thickness; cortical thickness is most reduced in the depressed participants with a history of abuse.
Using the matched sample, significant interaction effects were observed in the inferior frontalis gyrus (including the pars opercularis, and the pars triangularis), the inferior parietal gyrus, supramarginal gyrus, and temporal pole, highlighting these regions despite the smaller sample size (see Table SI4).
Discussion
To deepen the understanding of the interplay between childhood abuse and depressive symptoms, this study investigated the interaction of both risk factors on cortical thickness in a large population-based sample. This study adds essential results to the research domain examining the effects of childhood abuse on mental health by focusing exclusively on childhood abuse interacting with depressive symptoms, extending the commonly used covariate list, and analysing both healthy and depressed, in addition to abused and non-abused participants.
The interaction of childhood abuse and depressive symptoms on whole-brain cortical thickness
In the present study, only in abused participants, lower whole-brain cortical thickness was associated with more severe depressive symptoms.
Based on our results, studies analysing the effects of childhood abuse on cortical thickness should not neglect the impact of depressive symptoms. Against our assumption, abused participants without depressive symptoms showed an increased whole-brain cortical thickness in contrast to non-depressed and non-abused participants in our sample. This finding is in congruence with a recent study reporting increased cortical thickness in non-depressed (compared to depressed), abused participants in the orbitofrontal cortex [44]. Here, we found a significant interaction in the lateral orbitofrontal gyri. Interestingly, Habets et al. [45] found a similar effect in patients with schizophrenia and their healthy siblings, whereas both experienced childhood maltreatment. They found a reduction in cortical thickness in the patient group and an increase of cortical thickness in the healthy sibling group. These studies show, consistent to our data, that comparing healthy participants without a history of childhood abuse with both abused and depressed participants does display only a small reflection of the whole interaction context.
On the other hand, the variable of childhood abuse appears to be a relevant moderator when analysing the effects of depressive symptoms on cortical thickness. Thus, studies analysing the association between depression and cortical thickness might be more likely to find significant results if they do not control for childhood abuse or have a high proportion of abused participants, which might be especially true for patient samples [5]. Our results suggest that depressive symptoms alone, do not significantly influence the whole-brain cortical thickness, as correspondingly reported [13]. Another explanation suggests that the effect sizes are too small to detect the influence. Analysing the effects of depression on cortical thickness, critical parameters in neurological alterations are the age of onset and the severity of depression (either recurrent or severity of symptoms) [14]. We could emphasize that moderate to severe depressive symptoms are a relevant factor concerning neurological alteration by being significantly associated with thinner cortices (compared to no symptoms) combined with a history of abuse. Future research could combine these factors to deepen the understanding of the influence of depressive symptoms on cortical thickness.
The influence of childhood abuse and depressive symptoms on cortical regions
Our results demonstrated a widespread effect of the interaction of childhood abuse and depressive symptoms on cortical thickness. Cortical regions with the highest interaction effects are involved in emotional processing, social cognition, and sensory processing. Cortical thickness of the inferior parietal lobe and the inferior parietal lobe was significantly associated with the childhood abuse-depression-interaction effect in our study. The inferior parietal lobe, divided into the supramarginal gyrus and the angular gyrus, is crucial for overcoming emotional egocentricity avoiding biased social judgments [46]. The inferior parietal lobe was described to handle visuospatial information, be involved in perspective-taking and emotional processing through distancing [47, 48]. Moreover, we found associations with the inferior frontal gyrus, involved in response inhibition and attention control as part of the executive control [49, 50] and previously reported to be thinner in abused participants [51, 52]. Similarly, the cortical thickness of the temporal pole was associated with childhood maltreatment, and physical abuse in particular, before [53, 54]. It is essential in processing social cognition, naming the theory of mind [55]. Furthermore, we found reduced cortical thickness in visual cortex structures, such as the pericalcarine and the later occipital cortex. Earlier studies found reduced grey matter volume, cortical volume, and cortical thickness in the visual cortex in participants with a history of abuse [10, 56,57,58]. However, associations between these regional reductions and depressive symptoms were not explicitly reported before (e.g., by Schmaal et al. [14]). Thus, we conclude that these reductions show explicitly relevant interaction effects of childhood abuse and depressive symptoms.
Consequences of childhood abuse on cortical thickness
Every cortical region processes certain qualities of a sensory stimulus [59]. Studies analysing the cellular architecture of the cortex demonstrate that the cortical thickness is contingent on the number of neurons, glia provision, and the neuronal structure [60, 61]. Also, differences in cortical thickness are associated with the structural hierarchical level of processing the stimulus [59]. In the group of participants with no depressive symptoms, the present study found larger cortical thickness in abused compared to non-abused subjects. An explanation for our findings could be the sensitisation theory of Heim et al. (2008) hypothesising that childhood abuse sensitises the pituitary and a counter-adjustment is needed to compensate [62]. In line with this, Teicher et al. [5] argued that maltreated subjects could also differ in brain changes which help them compensate [5]. Previous studies found associations between increased cortical thickness and resilience [73].
Experiencing early life stress, such as childhood abuse, is a severe intrusion in the early development stage [5]. This intrusion can interfere with the neuronal development [63,64,65]. Consequently, cerebral structures can be prone to future stressful influences [66, 67]. Based on our results, we hypothesise that childhood abuse is a relevant biological disposition that interacts with the current state of psychopathology. Thus, structural brain alterations might follow a different pattern in depressed participants with a history of abuse compared to participants without a history of abuse [5]. Certainly, these results need to be carefully interpreted as other factors such as cognitive training [68], cortical asymmetry [69], and cellular architecture [59, 61] affect the cortical thickness.
Lim et al. [15] argued that the experience of abuse during childhood may lead to a reduction in synapses to protect the child from a hostile environment. These alterations could affect emotional and social processing in depressed patients resulting in a lower treatment outcome. In line with that, meta-analyses revealed that depressed patients with childhood adversity showed reduced benefit from treatment [19, 70]. Future research would be of prime importance to investigate if these abused, and treatment-resistant patients also have remarkably low cortical thickness. Furthermore, the research could focus on how these associations might change with pharmacological or psychotherapeutic therapy and whether these alterations are reversible. We focussed on the consequences of childhood abuse as it is well described in the literature and has a tremendous effect on the cortex [11, 52, 56]. However, it would be important to analyse the interaction effect of neglect and depressive symptoms interacting.
Limitations
Despite our careful study design, there are several limitations worth noticing. First, we used self-report measures to assess the depressive symptoms and to assess the history of childhood abuse. The PHQ-9 is a validated measurement, but it does not replace a formal psychiatric anamnesis. Also, we focussed only on current depressive symptoms and did not include the influence of lifetime diagnosis of MDD or other psychiatric diagnoses. Therefore, we cannot eliminate other psychiatric diagnoses, which could influence our results. As we did not analyse a psychiatric cohort, we assume that psychiatric symptomatology is low. The CTQ is a self-reported measure that does not assess at which age the abuse or neglect happened. Further, participants with a history of abuse could intentionally not report these traumata due to avoidance or repression.
Second, we focused on abuse-related effects but incorporated participants with the exposure of abuse and neglect. Hence, we cannot differentiate between effects due to childhood abuse or childhood neglect. Childhood abuse and childhood neglect are strongly correlated, and childhood maltreatment is associated with depressive symptoms. However, as we excluded participants exclusively reporting childhood neglect but included participants exclusively reporting childhood abuse, our results are more likely to reflect the effects of childhood abuse. We decided to include subjects with a history of neglect and abuse, as there is a considerable overlap of subjects who experienced both forms of maltreatment [71]. Future studies are needed to differentiate between the effects of abuse and neglect.
Third, the distribution of the abuse and neglect severity is not equally distributed across the depression groups (see Table SI1). As the sample was recruited from the general population and associations between childhood maltreatment and depressive symptoms are well described [19, 20], this is an expected, data immanent issue. Nevertheless, we cannot rule out that this association might impact the results and future studies are needed matching abuse and neglect severity in depressed and non-depressed participants to validate our results.
Fourth, due to the cross-sectional study design, our results do not allow any causal conclusion. Further, although the analysed study sample was large and a large set of potential covariates was included, we did not collect a replication sample. In addition, age-range and sex differences often moderate effects on cortical thickness [72, 73]. We tried to rule out these effects by including both variables as covariates in our statistical models and analysing the results within a sample matched for sex, age, and depression level. We did not adjust for potential comorbidities (such as hypertension or diabetes) as we focus on the general population in which comorbid medical conditions are common. Finally, our study sample is not entirely independent from the ENIGMA consortium, but we included different covariates in our analysis.
Conclusion
This study investigated the interaction of childhood abuse and depressive symptoms on cortical thickness. Based on a large population-based sample, only in the presence of childhood abuse did depressive symptoms influence the whole-brain cortical thickness. This might explain some of the inconsistencies reported in previous studies. The interaction between childhood abuse and depressive symptoms was associated with a widespread reduction in cortical thickness in regions involved in emotional processing, social cognition, and sensory processing. Larger cortical thickness, in abused subjects without depressive symptoms, might act compensatory and thus reflect resilience against depressive symptoms. Our results support the hypothesis that childhood abuse is moderating the associations between cortical thickness and depressive symptoms, particularly in participants with more severe depressive symptoms. Further research could focus on how different treatments can affect brain alterations associated with childhood abuse and depressive symptoms and whether those are reversible.
Availability of data and materials
SHIP data are applicable via fvcm.med.uni-greifswald.de.
Code availability
Not applicable.
References
American Professional Society on the Abuse of Children (APSAC) (1996) Psychosocial evaluation of suspected psychological maltreatment in children and adolescents. American Professional Society on the Abuse of Children, New York
Witt A, Brown RC, Plener PL et al (2017) Child maltreatment in Germany: Prevalence rates in the general population. Child Adolesc Psychiatry Ment Health 11:1–9. https://doi.org/10.1186/s13034-017-0185-0
Danese A, Caspi A, Williams B et al (2011) Biological embedding of stress through inflammation processes in childhood. Mol Psychiatry 16:244–246. https://doi.org/10.1038/mp.2010.5
Danese A, McEwen BS (2012) Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav 106:29–39. https://doi.org/10.1016/j.physbeh.2011.08.019
Teicher MH, Samson JA, Anderson CM, Ohashi K (2016) The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci 17:652–666. https://doi.org/10.1038/nrn.2016.111
Wegman HL, Stetler C (2009) A meta-analytic review of the effects of childhood abuse on medical outcomes in adulthood. Psychosom Med. https://doi.org/10.1097/PSY.0b013e3181bb2b46
Maniglio R (2010) Child sexual abuse in the etiology of depression: a systematic review of reviews. Depress Anxiety 27:631–642. https://doi.org/10.1002/da.20687
Fritch AM, Mishkind M, Reger MA, Gahm GA (2010) The impact of childhood abuse and combat-related trauma on postdeployment adjustment. J Trauma Stress. https://doi.org/10.1002/jts.20520
Carballedo A, Lisiecka D, Fagan A et al (2012) Early life adversity is associated with brain changes in subjects at family risk for depression. World J Biol Psychiatry 13:569–578. https://doi.org/10.3109/15622975.2012.661079
Tomoda A, Polcari A, Anderson CM, Teicher MH (2012) Reduced visual cortex gray matter volume and thickness in young adults who witnessed domestic violence during childhood. PLoS ONE 7:1–11. https://doi.org/10.1371/journal.pone.0052528
Heim CM, Mayberg HS, Mletzko T et al (2013) Decreased cortical representation of genital somatosensory field after childhood sexual abuse. Am J Psychiatry 170:616–623
McLaughlin KA, Sheridan MA, Lambert HK (2014) Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev 47:578–591
Tozzi L, Garczarek L, Janowitz D et al (2020) Interactive impact of childhood maltreatment, depression, and age on cortical brain structure: mega-analytic findings from a large multi-site cohort. Psychol Med 50:1020–1031. https://doi.org/10.1017/S003329171900093X
Schmaal L, Hibar DP, Sämann PG et al (2017) Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry 22:900–909. https://doi.org/10.1038/mp.2016.60
Lim L, Hart H, Mehta M et al (2018) Grey matter volume and thickness abnormalities in young people with a history of childhood abuse. Psychol Med. https://doi.org/10.1017/S0033291717002392
Sheikh MA (2017) Confounding and statistical significance of indirect effects: childhood adversity, education, smoking, and anxious and depressive symptomatology. Front Psychol. https://doi.org/10.3389/fpsyg.2017.01317
Anderson SE, Cohen P, Naumova EN et al (2007) Adolescent obesity and risk for subsequent major depressive disorder and anxiety disorder: prospective evidence. Psychosom Med. https://doi.org/10.1097/PSY.0b013e31815580b4
Hart H, Rubia K (2012) Neuroimaging of child abuse: a critical review. Front Hum Neurosci 6:1–24. https://doi.org/10.3389/fnhum.2012.00052
Klein DN, Arnow BA, Barkin JL et al (2009) Early adversity in chronic depression: clinical correlates and response to pharmacotherapy. Depress Anxiety. https://doi.org/10.1002/da.20577
Youssef NA, Green KT, Dedert EA et al (2013) Exploration of the influence of childhood trauma, combat exposure, and the resilience construct on depression and suicidal ideation among U.S. Iraq/Afghanistan Era Military Personnel and Veterans. Arch Suicide Res 17:106–122. https://doi.org/10.1080/13811118.2013.776445
Völzke H (2012) Study of Health in Pomerania (SHIP). Bundesgesundheitsblatt Gesundheitsforsch Gesundheitsschutz 55:790–794. https://doi.org/10.1007/s00103-012-1483-6
Hegenscheid K, Seipel R, Schmidt CO et al (2013) Potentially relevant incidental findings on research whole-body MRI in the general adult population: frequencies and management. Eur Radiol 23:816–826. https://doi.org/10.1007/s00330-012-2636-6
Hegenscheid K, Kühn J, Völzke H et al (2009) Whole-body magnetic resonance imaging of healthy volunteers: pilot study results from the population-based SHIP study. RöFo Fortschritte auf dem Gebiet der Röntgenstrahlen und der Bildgeb Verfahren 181:748–759. https://doi.org/10.1055/s-0028-1109510
Baumeister SE, Alte D, Meyer C, John U (2005) Riskanter Alkoholkonsum und alkoholbezogene Störungen in Vorpommern: Die Studie „Leben und Gesundheit in Vorpommern” (SHIP) und der Bundesgesundheitssurvey 1998 im Vergleich. Das Gesundheitswes 67:39–47. https://doi.org/10.1055/s-2004-813829
ACT-Index (2007) Anatomisch- therapeutisch- chemische Klassifikation mit Tagesdosen. Amtliche Fassung des ATC-Index mit DD-Angaben für Deutschland
Wingenfeld K, Spitzer C, Mensebach C et al (2010) Die deutsche version des Childhood Trauma Questionnaire (CTQ): Erste Befunde zu den psychometrischen Kennwerten. PPmP- Psychother Psychosom Medizinische Psychol 60:e13–e13. https://doi.org/10.1055/s-0030-1253494
Bernstein DP, Stein JA, Newcomb MD et al (2003) Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl 27:169–190. https://doi.org/10.1016/S0145-2134(02)00541-0
Bernstein DP, Fink L, Handelsman L et al (1994) Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry 151:1132–1136. https://doi.org/10.1176/ajp.151.8.1132
Klinitzke G, Romppel M, Häuser W et al (2012) Die deutsche Version des Childhood Trauma Questionnaire (CTQ) psychometrische Eigenschaften in einer bevölkerungsrepräsentativen Stichprobe. PPmP Psychother Psychosom Medizinische Psychol. https://doi.org/10.1055/s-0031-1295495
Kroenke K, Spitzer RL (2002) The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann 32:509–515. https://doi.org/10.3928/0048-5713-20020901-06
Gräfe K, Zipfel S, Herzog W, Löwe B (2004) Screening psychischer Störungen mit dem „Gesundheitsfragebogen für Patienten (PHQ-D)“. Diagnostica. https://doi.org/10.1026/0012-1924.50.4.171
Ségonne F, Dale AM, Busa E et al (2004) A hybrid approach to the skull stripping problem in MRI. Neuroimage 22:1060–1075. https://doi.org/10.1016/j.neuroimage.2004.03.032
Sled JG, Zijdenbos AP, Evans AC (1998) A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17:87–97. https://doi.org/10.1109/42.668698
Fischl B, Liu A, Dale AM (2001) Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging 20:70–80. https://doi.org/10.1109/42.906426
Segonne F, Pacheco J, Fischl B (2007) Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging 26:518–529. https://doi.org/10.1109/TMI.2006.887364
Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis. Neuroimage 9:179–194. https://doi.org/10.1006/nimg.1998.0395
Dale AM, Sereno MI (1993) Improved localizadon of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci 5:162–176. https://doi.org/10.1162/jocn.1993.5.2.162
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci 97:11050–11055. https://doi.org/10.1073/pnas.200033797
Fischl B, Sereno MI, Tootell RBH, Dale AM (1999) High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 8:272–284. https://doi.org/10.1002/(sici)1097-0193(1999)8:4%3c272::aid-hbm10%3e3.0.co;2-4
Desikan RS, Ségonne F, Fischl B et al (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980. https://doi.org/10.1016/j.neuroimage.2006.01.021
Fischl B (2004) Automatically parcellating the human cerebral cortex. Cereb Cortex 14:11–22. https://doi.org/10.1093/cercor/bhg087
Hochberg Y, Benjamini Y (1990) More powerful procedures for multiple significance testing. Stat Med. https://doi.org/10.1002/sim.4780090710
RStudio Team (2015) RStudio: Integrated Development for R. RStudio, Inc., Boston
Saleh A, Potter GG, McQuoid DR et al (2017) Effects of early life stress on depression, cognitive performance and brain morphology. Psychol Med 47:171–181. https://doi.org/10.1017/S0033291716002403
Habets P, Marcelis M, Gronenschild E et al (2011) Reduced cortical thickness as an outcome of differential sensitivity to environmental risks in schizophrenia. Biol Psychiatry 69:487–494. https://doi.org/10.1016/j.biopsych.2010.08.010
Silani G, Lamm C, Ruff CC, Singer T (2013) Right supramarginal gyrus is crucial to overcome emotional egocentricity bias in social judgments. J Neurosci 33:15466–15476. https://doi.org/10.1523/JNEUROSCI.1488-13.2013
Koenigsberg HW, Fan J, Ochsner KN et al (2010) Neural correlates of using distancing to regulate emotional responses to social situations. Neuropsychologia. https://doi.org/10.1016/j.neuropsychologia.2010.03.002
Parkinson C, Liu S, Wheatley T (2014) A common cortical metric for spatial, temporal, and social distance. J Neurosci 34:1979–1987. https://doi.org/10.1523/JNEUROSCI.2159-13.2014
Hampshire A, Chamberlain SR, Monti MM et al (2010) The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. https://doi.org/10.1016/j.neuroimage.2009.12.109
Cole MW, Schneider W (2007) The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage. https://doi.org/10.1016/j.neuroimage.2007.03.071
Gold AL, Sheridan MA, Peverill M et al (2016) Childhood abuse and reduced cortical thickness in brain regions involved in emotional processing. J Child Psychol Psychiatry 57:1154–1164. https://doi.org/10.1111/jcpp.12630
Lim L, Radua J, Rubia K (2014) Gray matter abnormalities in childhood maltreatment: a voxelwise metaanalysis. Am J Psychiatry. https://doi.org/10.1176/appi.ajp.2014.13101427
Teicher MH, Anderson CM, Ohashi K, Polcari A (2014) Childhood maltreatment: altered network centrality of cingulate, precuneus, temporal pole and insula. Biol Psychiatry 76:297–305. https://doi.org/10.1016/j.biopsych.2013.09.016
Hanson JL, Chung MK, Avants BB et al (2010) Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J Neurosci 30:7466–7472. https://doi.org/10.1523/JNEUROSCI.0859-10.2010
Ross LA, Olson IR (2010) Social cognition and the anterior temporal lobes. Neuroimage 49:3452–3462. https://doi.org/10.1016/j.neuroimage.2009.11.012
Tomoda A, Navalta CP, Polcari A et al (2009) Childhood sexual abuse is associated with reduced gray matter volume in visual cortex of young women. Biol Psychiatry 66:642–648. https://doi.org/10.1016/j.biopsych.2009.04.021
Edmiston EE, Wang F, Mazure CM et al (2011) Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Arch Pediatr Adolesc Med 165:1069–1077. https://doi.org/10.1001/archpediatrics.2011.565
Lim L, Hart H, Mehta M et al (2018) Grey matter volume and thickness abnormalities in young people with a history of childhood abuse. Psychol Med 48:1034–1046. https://doi.org/10.1017/S0033291717002392
Wagstyl K, Ronan L, Goodyer IM, Fletcher PC (2015) Cortical thickness gradients in structural hierarchies. Neuroimage 111:241–250. https://doi.org/10.1016/j.neuroimage.2015.02.036
Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B (2008) Neocortical glial cell numbers in human brains. Neurobiol Aging 29:1754–1762. https://doi.org/10.1016/j.neurobiolaging.2007.04.013
La Fougère C, Grant S, Kostikov A et al (2011) Where in-vivo imaging meets cytoarchitectonics: the relationship between cortical thickness and neuronal density measured with high-resolution [18F]flumazenil-PET. Neuroimage 56:951–960. https://doi.org/10.1016/j.neuroimage.2010.11.015
Heim C, Newport DJ, Mletzko T et al (2008) The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology 33:693–710. https://doi.org/10.1016/j.psyneuen.2008.03.008
Gee DG, Gabard-Durnam LJ, Flannery J et al (2013) Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.1307893110
Andersen SL, Tomada A, Vincow ES et al (2008) Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. https://doi.org/10.1176/jnp.2008.20.3.292
Teicher MH, Khan A (2019) Childhood maltreatment, cortical and amygdala morphometry, functional connectivity, laterality, and psychopathology. Child Maltreat. https://doi.org/10.1177/1077559519870845
Shonkoff JP, Boyce WT, McEwen BS (2009) Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. J Am Med Assoc 301:2252–2259
Oldehinkel AJ, Ormel J, Verhulst FC, Nederhof E (2014) Childhood adversities and adolescent depression: a matter of both risk and resilience. Dev Psychopathol 26:1067–1075
Jiang L, Cao X, Li T et al (2016) Cortical thickness changes correlate with cognition changes after cognitive training: evidence from a Chinese community study. Front Aging Neurosci 8:1–8. https://doi.org/10.3389/fnagi.2016.00118
Zhou D, Lebel C, Evans A, Beaulieu C (2013) Cortical thickness asymmetry from childhood to older adulthood. Neuroimage 83:66–74. https://doi.org/10.1016/j.neuroimage.2013.06.073
Nanni V, Uher R, Danese A (2012) Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am J Psychiatry. https://doi.org/10.1176/appi.ajp.2011.11020335
Edwards VJ, Holden GW, Felitti VJ, Anda RF (2003) Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry. https://doi.org/10.1176/appi.ajp.160.8.1453
Luders E, Narr KL, Thompson PM et al (2006) Gender effects on cortical thickness and the influence of scaling. Hum Brain Mapp. https://doi.org/10.1002/hbm.20187
Hurtz S, Woo E, Kebets V et al (2014) Age effects on cortical thickness in cognitively normal elderly individuals. Dement Geriatr Cogn Dis Extra. https://doi.org/10.1159/000362872
Funding
Open Access funding enabled and organized by Projekt DEAL. The Study of Health in Pomerania (SHIP) is part of the Community Medicine Research net (CMR) (http://www.medizin.uni-greifswald.de/icm) of the University Medicine Greifswald, which is funded by the Federal Ministry of Education and Research (01ZZ9603, 01ZZ0103, and 01ZZ0403), the Ministry of Cultural Affairs and the Social Ministry of the Federal State of Mecklenburg-West Pomerania was funded by a joint grant from Siemens Healthineers (Erlangen, Germany) and the Federal State of Mecklenburg-West Pomerania. SF was supported by the EU-JPND Funding for BRIDGET (FKZ:01ED1615) and the National Institute of Health (NIH) grant AG059421.
Author information
Authors and Affiliations
Contributions
SV: conceptualization, methodology, formal analysis, investigation, original draft. SF: MRI data processing, review & editing. JKK: conceptualization, review & editing. DJ: review & editing. KW: investigation, review & editing. RB: investigation, review & editing. HV: investigation, review & editing. HJG: conceptualization, investigation, review & editing.
Corresponding author
Ethics declarations
Conflict of interest
HJG has received travel grants and speakers’ honoraria from Fresenius Medical Care, Neuraxpharm, Servier and Janssen Cilag as well as research funding from Fresenius Medical Care.
Ethical approval
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Informed consent
All participants provided written informed consent.
Consent to participate
The institutional review board of the University Greifswald approved SHIP. Examinations and assessments have been conducted by the declaration of Helsinki, including written informed consent from all participants.
Consent to publish
All authors have read and approved the manuscript for publication. The publication of the used data is consented by a data application.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Voss, S., Frenzel, S., Klinger-König, J. et al. Interaction of childhood abuse and depressive symptoms on cortical thickness: a general population study. Eur Arch Psychiatry Clin Neurosci 272, 1523–1534 (2022). https://doi.org/10.1007/s00406-022-01387-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-022-01387-8