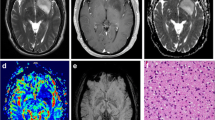
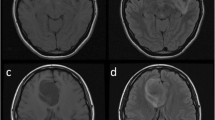
Abstract
Objectives
The purpose of this study was to investigate the clinical utility of the sinuous, wave-like intratumoral-wall (SWITW) sign on T2WI in diagnosing isocitrate dehydrogenase (IDH) mutant and 1p/19q codeleted (IDHmut-Codel) oligodendrogliomas, for which a relatively conservative resection strategy might be sufficient due to a better response to chemoradiotherapy and favorable prognosis.
Methods
Imaging data from consecutive adult patients with diffuse lower-grade gliomas (LGGs, histological grades 2–3) in Beijing Tiantan Hospital (December 1, 2013, to October 31, 2021, BTH set, n = 711) and the Cancer Imaging Archive (TCIA) LGGs set (n = 117) were used to develop and validate our findings. Two independent observers assessed the SWITW sign and some well-reported discriminative radiological features to establish a practical diagnostic strategy.
Results
The SWITW sign showed satisfying sensitivity (0.684 and 0.722 for BTH and TCIA sets) and specificity (0.938 and 0.914 for BTH and TCIA sets) in defining IDHmut-Codels, and the interobserver agreement was substantial (κ 0.718 and 0.756 for BTH and TCIA sets). Compared to calcification, the SWITW sign improved the sensitivity by 0.28 (0.404 to 0.684) in the BTH set, and 81.0% (277/342) of IDHmut-Codel cases demonstrated SWITW and/ or calcification positivity. Combining the SWITW sign, calcification, low ADC values, and other discriminative features, we established a concise and reliable diagnostic protocol for IDHmut-Codels.
Conclusions
The SWITW sign was a sensitive and specific imaging biomarker for IDHmut-Codels. The integrated protocol provided an explicable, efficient, and reproducible method for precise preoperative diagnosis, which was essential to guide individualized surgical plan-making.
Key Points
• The SWITW sign was a sensitive and specific imaging biomarker for IDHmut-Codel oligodendrogliomas.
• The SWITW sign was more sensitive than calcification and an integrated strategy could improve diagnostic sensitivity for IDHmut-Codel oligodendrogliomas.
• Combining SWITW, calcification, low ADC values, and other discriminative features could make a precise preoperative diagnosis for IDHmut-Codel oligodendrogliomas.




Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area under the curve
- BTH:
-
Beijing Tiantan Hospital
- DT:
-
Decision tree
- FLAIR:
-
Fluid-attenuated inversion-recovery
- GBDT:
-
Gradient boosting decision tree
- hyperFLAIRrim:
-
Hyperintense FLAIR rim
- IDH:
-
Isocitrate dehydrogenase
- LASSO:
-
Least absolute shrinkage and selection operator
- LGG:
-
Lower-grade glioma
- MGMT:
-
O6-methylguanine-DNA methyltransferase
- NPV:
-
Negative predictive value
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- PPV:
-
Positive predictive value
- RF:
-
Random forests
- ROC:
-
Receiver operating characteristic curve
- SVM:
-
Support vector machine
- SWITW:
-
Sinuous wave-like intratumoral-wall feature on T2WI
- T2FM:
-
T2-FLAIR mismatch
- VASARI:
-
Visually Accessible Rembrandt Images
- VOI:
-
Volumes of interest
- WHO:
-
World Health Organization
References
Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS (2020) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro Oncol 22:iv1–iv96. https://doi.org/10.1093/neuonc/noaa200
Louis DN, Perry A, Wesseling P et al (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251. https://doi.org/10.1093/neuonc/noab106
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Rossi M, Ambrogi F, Gay L et al (2019) Is supratotal resection achievable in low-grade gliomas? Feasibility, putative factors, safety, and functional outcome. J Neurosurg 132:1692–1705. https://doi.org/10.3171/2019.2.JNS183408
Cluceru J, Interian Y, Phillips JJ et al (2022) Improving the noninvasive classification of glioma genetic subtype with deep learning and diffusion-weighted imaging. Neuro Oncol 24:639–652. https://doi.org/10.1093/neuonc/noab238
Li M, Ren X, Chen X et al (2022) Combining hyperintense FLAIR rim and radiological features in identifying IDH mutant 1p/19q non-codeleted lower-grade glioma. Eur Radiol. https://doi.org/10.1007/s00330-021-08500-w
Yan H, Parsons DW, Jin G et al (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360:765–773. https://doi.org/10.1056/NEJMoa0808710
Aibaidula A, Chan AK, Shi Z et al (2017) Adult IDH wild-type lower-grade gliomas should be further stratified. Neuro Oncol 19:1327–1337. https://doi.org/10.1093/neuonc/nox078
Baldock AL, Yagle K, Born DE et al (2014) Invasion and proliferation kinetics in enhancing gliomas predict IDH1 mutation status. Neuro Oncol 16:779–786. https://doi.org/10.1093/neuonc/nou027
Wang P, Luo C, Hong P, Rui W, Wu S (2021) The role of surgery in IDH-wild-type lower-grade gliomas: threshold at a high extent of resection should be pursued. Neurosurgery 88:1136–1144. https://doi.org/10.1093/neuros/nyab052
Wijnenga MMJ, French PJ, Dubbink HJ et al (2018) The impact of surgery in molecularly defined low-grade glioma: an integrated clinical, radiological, and molecular analysis. Neuro Oncol 20:103–112. https://doi.org/10.1093/neuonc/nox176
van Lent DI, van Baarsen KM, Snijders TJ, Robe P (2020) Radiological differences between subtypes of WHO 2016 grade II-III gliomas: a systematic review and meta-analysis. Neurooncol Adv 2:vdaa044. https://doi.org/10.1093/noajnl/vdaa044
Horbinski C, McCortney K, Stupp R (2021) MGMT promoter methylation is associated with patient age and 1p/19q status in IDH-mutant gliomas. Neuro Oncol 23:858–860. https://doi.org/10.1093/neuonc/noab039
Li M, Dong G, Zhang W et al (2021) Combining MGMT promoter pyrosequencing and protein expression to optimize prognosis stratification in glioblastoma. Cancer Sci 112:3699–3710. https://doi.org/10.1111/cas.15024
Li M, Ren X, Dong G et al (2021) Distinguishing pseudoprogression from true early progression in isocitrate dehydrogenase wild-type glioblastoma by interrogating clinical, radiological, and molecular features. Front Oncol 11:627325. https://doi.org/10.3389/fonc.2021.627325
van der Voort SR, Incekara F, Wijnenga MM et al (2019) Predicting the 1p/19q co-deletion status of presumed low grade glioma with an externally validated machine learning algorithm. Clin Cancer Res. https://doi.org/10.1158/1078-0432.Ccr-19-1127
Smits M, van den Bent MJ (2017) Imaging correlates of adult glioma genotypes. Radiology 284:316–331. https://doi.org/10.1148/radiol.2017151930
Smits M (2016) Imaging of oligodendroglioma. Br J Radiol 89:20150857. https://doi.org/10.1259/bjr.20150857
van den Bent MJ, Smits M, Kros JM, Chang SM (2017) Diffuse infiltrating oligodendroglioma and astrocytoma. J Clin Oncol 35:2394–2401. https://doi.org/10.1200/JCO.2017.72.6737
Yushkevich P, Piven J, Hazlett H et al (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31:1116–1128. https://doi.org/10.1016/j.neuroimage.2006.01.015
Xiong J, Tan W, Wen J et al (2016) Combination of diffusion tensor imaging and conventional MRI correlates with isocitrate dehydrogenase 1/2 mutations but not 1p/19q genotyping in oligodendroglial tumours. Eur Radiol 26:1705–1715. https://doi.org/10.1007/s00330-015-4025-4
Tan WL, Huang WY, Yin B, Xiong J, Wu JS, Geng DY (2014) Can diffusion tensor imaging noninvasively detect IDH1 gene mutations in astrogliomas? A retrospective study of 112 cases. AJNR Am J Neuroradiol 35:920–927. https://doi.org/10.3174/ajnr.A3803
van den Bent MJ, Wefel JS, Schiff D et al (2011) Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol 12:583–593. https://doi.org/10.1016/S1470-2045(11)70057-2
Wen PY, Chang SM, Van den Bent MJ, Vogelbaum MA, Macdonald DR, Lee EQ (2017) Response assessment in neuro-oncology clinical trials. J Clin Oncol 35:2439–2449. https://doi.org/10.1200/JCO.2017.72.7511
Wijnenga MMJ, van der Voort SR, French PJ et al (2019) Differences in spatial distribution between WHO 2016 low-grade glioma molecular subgroups. Neurooncol Adv 1:vdz001. https://doi.org/10.1093/noajnl/vdz001
Larjavaara S, Mäntylä R, Salminen T et al (2007) Incidence of gliomas by anatomic location. Neuro Oncol 9:319–325. https://doi.org/10.1215/15228517-2007-016
Rossi M, Gay L, Ambrogi F et al (2021) Association of supratotal resection with progression-free survival, malignant transformation, and overall survival in lower-grade gliomas. Neuro Oncol 23:812–826. https://doi.org/10.1093/neuonc/noaa225
Molinaro A, Hervey-Jumper S, Morshed R et al (2020) Association of maximal extent of resection of contrast-enhanced and non-contrast-enhanced tumor with survival within molecular subgroups of patients with newly diagnosed glioblastoma. JAMA Oncol 6:495–503. https://doi.org/10.1001/jamaoncol.2019.6143
Cahill DP (2021) Extent of resection of glioblastoma: a critical evaluation in the molecular era. Neurosurg Clin N Am 32:23–29. https://doi.org/10.1016/j.nec.2020.09.006
Mur P, Mollejo M, Ruano Y et al (2013) Codeletion of 1p and 19q determines distinct gene methylation and expression profiles in IDH-mutated oligodendroglial tumors. Acta Neuropathol 126:277–289. https://doi.org/10.1007/s00401-013-1130-9
Kawaguchi T, Sonoda Y, Shibahara I et al (2016) Impact of gross total resection in patients with WHO grade III glioma harboring the IDH 1/2 mutation without the 1p/19q co-deletion. J Neurooncol 129:505–514. https://doi.org/10.1007/s11060-016-2201-2
Ding X, Wang Z, Chen D et al (2018) The prognostic value of maximal surgical resection is attenuated in oligodendroglioma subgroups of adult diffuse glioma: a multicenter retrospective study. J Neurooncol 140:591–603. https://doi.org/10.1007/s11060-018-2985-3
WHO Classification of Tumours Editorial Board. Central nervous system tumours. Lyon (France): International Agency for Research on Cancer; 2021. (WHO classification of tumours series, 5th ed; vol 6). https://publications.iarc.fr/601
Jenkinson MD, du Plessis DG, Smith TS, Joyce KA, Warnke PC, Walker C (2006) Histological growth patterns and genotype in oligodendroglial tumours: correlation with MRI features. Brain 129:1884–1891. https://doi.org/10.1093/brain/awl108
Khalid L, Carone M, Dumrongpisutikul N et al (2012) Imaging characteristics of oligodendrogliomas that predict grade. AJNR Am J Neuroradiol 33:852–857. https://doi.org/10.3174/ajnr.A2895
Kiroglu Y, Calli C, Karabulut N, Oncel C (2010) Intracranial calcifications on CT. Diagn Interv Radiol 16:263–269. https://doi.org/10.4261/1305-3825.DIR.2626-09.1
Saade C, Najem E, Asmar K, Salman R, El Achkar B, Naffaa L (2019) Intracranial calcifications on CT: an updated review. J Radiol Case Rep 13:1–18. https://doi.org/10.3941/jrcr.v13i8.3633
Zhao K, Sun G, Wang Q et al (2021) The Diagnostic value of conventional MRI and CT features in the identification of the IDH1-mutant and 1p/19q co-deletion in WHO grade II gliomas. Acad Radiol 28:e189–e198. https://doi.org/10.1016/j.acra.2020.03.008
Saito T, Muragaki Y, Maruyama T et al (2016) Calcification on CT is a simple and valuable preoperative indicator of 1p/19q loss of heterozygosity in supratentorial brain tumors that are suspected grade II and III gliomas. Brain Tumor Pathol 33:175–182. https://doi.org/10.1007/s10014-016-0249-5
Patel SH, Poisson LM, Brat DJ et al (2017) T2-FLAIR mismatch, an imaging biomarker for IDH and 1p/19q status in lower-grade gliomas: a TCGA/TCIA project. Clin Cancer Res 23:6078–6085. https://doi.org/10.1158/1078-0432.CCR-17-0560
Broen MPG, Smits M, Wijnenga MMJ et al (2018) The T2-FLAIR mismatch sign as an imaging marker for non-enhancing IDH-mutant, 1p/19q-intact lower-grade glioma: a validation study. Neuro Oncol 20:1393–1399. https://doi.org/10.1093/neuonc/noy048
Foltyn M, Nieto Taborda KN, Neuberger U et al (2020) T2/FLAIR-mismatch sign for noninvasive detection of IDH-mutant 1p/19q non-codeleted gliomas: validity and pathophysiology. Neurooncol Adv 2:vdaa004. https://doi.org/10.1093/noajnl/vdaa004
Batchala PP, Muttikkal TJE, Donahue JH et al (2019) Neuroimaging-based classification algorithm for predicting 1p/19q-codeletion status in IDH-mutant lower grade gliomas. AJNR Am J Neuroradiol 40:426–432. https://doi.org/10.3174/ajnr.A5957
Throckmorton P, Graber JJ (2020) T2-FLAIR mismatch in isocitrate dehydrogenase mutant astrocytomas: Variability and evolution. Neurology 95:e1582–e1589. https://doi.org/10.1212/WNL.0000000000010324
Verburg N, Koopman T, Yaqub MM et al (2020) Improved detection of diffuse glioma infiltration with imaging combinations: a diagnostic accuracy study. Neuro Oncol 22:412–422. https://doi.org/10.1093/neuonc/noz180
Lee MK, Park JE, Jo Y, Park SY, Kim SJ, Kim HS (2020) Advanced imaging parameters improve the prediction of diffuse lower-grade gliomas subtype, IDH mutant with no 1p19q codeletion: added value to the T2/FLAIR mismatch sign. Eur Radiol 30:844–854. https://doi.org/10.1007/s00330-019-06395-2
Chen L, Liu M, Bao J et al (2013) The correlation between apparent diffusion coefficient and tumor cellularity in patients: a meta-analysis. PLoS One 8:e79008. https://doi.org/10.1371/journal.pone.0079008
Nam YK, Park JE, Park SY et al (2021) Reproducible imaging-based prediction of molecular subtype and risk stratification of gliomas across different experience levels using a structured reporting system. Eur Radiol 31:7374–7385. https://doi.org/10.1007/s00330-021-08015-4
Dono A, Ballester LY, Primdahl D, Esquenazi Y, Bhatia A (2021) IDH-mutant low-grade glioma: advances in molecular diagnosis, management, and future directions. Curr Oncol Rep 23:20. https://doi.org/10.1007/s11912-020-01006-6
Suh CH, Kim HS, Jung SC, Choi CG, Kim SJ (2019) Imaging prediction of isocitrate dehydrogenase (IDH) mutation in patients with glioma: a systemic review and meta-analysis. Eur Radiol 29:745–758. https://doi.org/10.1007/s00330-018-5608-7
Acknowledgments
The authors sincerely thank the patients and their families for their participation in the present study.
Funding sources
This study was supported by the Capital Funds for Health Improvement and Research (2020-2-1075 and 2022-2-1072) and the National Natural Science Foundation of China (81571632 and 81771309).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Xiaohui Ren, MD., PhD., and Song Lin, MD., PhD.
Conflict of interest
The authors declare no potential conflicts of interest.
Statistics and biometry
Mingxiao L and Jincheng W have significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• Multicenter study
Additional information
Xiaohui Ren and Song Lin were responsible for this study.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 2985 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, M., Wang, J., Chen, X. et al. The sinuous, wave-like intratumoral-wall sign is a sensitive and specific radiological biomarker for oligodendrogliomas. Eur Radiol 33, 4440–4452 (2023). https://doi.org/10.1007/s00330-022-09314-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09314-0