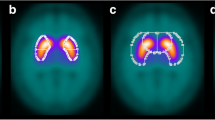
Abstract
Purpose
For the quantitative assessment of dopamine transporter (DAT) using [123I]FP-CIT single-photon emission computed tomography (SPECT) (DaTscan), anatomic standardization is preferable for achieving objective and user-independent quantification of striatal binding using a volume-of-interest (VOI) template. However, low accumulation of DAT in Parkinson’s disease (PD) would lead to a deformation error when using a DaTscan-specific template without any structural information. To avoid this deformation error, we applied computed tomography (CT) data obtained using SPECT/CT equipment to anatomic standardization.
Methods
We retrospectively analyzed DaTscan images of 130 patients with parkinsonian syndromes (PS), including 80 PD and 50 non-PD patients. First we segmented gray matter from CT images using statistical parametric mapping 12 (SPM12). These gray-matter images were then anatomically standardized using the diffeomorphic anatomical registration using exponentiated Lie algebra (DARTEL) algorithm. Next, DaTscan images were warped with the same parameters used in the CT anatomic standardization. The target striatal VOIs for decreased DAT in PD were generated from the SPM12 group comparison of 20 DaTscan images from each group. We applied these VOIs to DaTscan images of the remaining patients in both groups and calculated the specific binding ratios (SBRs) using nonspecific counts in a reference area. In terms of the differential diagnosis of PD and non-PD groups using SBR, we compared the present method with two other methods, DaTQUANT and DaTView, which have already been released as software programs for the quantitative assessment of DaTscan images.
Results
The SPM12 group comparison showed a significant DAT decrease in PD patients in the bilateral whole striatum. Of the three methods assessed, the present CT-guided method showed the greatest power for discriminating PD and non-PD groups, as it completely separated the two groups.
Conclusion
CT-guided anatomic standardization using the DARTEL algorithm is promising for the quantitative assessment of DaTscan images.





Similar content being viewed by others
References
Suwijn SR, van Boheemen CJ, de Haan RJ, Tissingh G, Booij J, de Bie RM. The diagnostic accuracy of dopamine transporter SPECT imaging to detect nigrostriatal cell loss in patients with Parkinson’s disease or clinically uncertain parkinsonism: a systematic review. EJNMMI Res. 2015;5:12. doi:10.1186/s13550-015-0087-1.
Politis M. Neuroimaging in Parkinson disease: from research setting to clinical practice. Nat Rev Neurol. 2014;10(12):708–22. doi:10.1038/nrneurol.2014.205.
Cummings JL, Henchcliffe C, Schaier S, Simuni T, Waxman A, Kemp P. The role of dopaminergic imaging in patients with symptoms of dopaminergic system neurodegeneration. Brain : J Neurol. 2011;134(Pt 11):3146–66. doi:10.1093/brain/awr177.
Gerasimou G, Costa DC, Papanastasiou E, Bostanjiopoulou S, Arnaoutoglou M, Moralidis E, et al. SPECT study with I-123-Ioflupane (DaTSCAN) in patients with essential tremor. Is there any correlation with Parkinson’s disease? Ann Nucl Med. 2012;26(4):337–44. doi:10.1007/s12149-012-0577-4.
Thiriez C, Itti E, Fenelon G, Evangelista E, Meignan M, Cesaro P, et al. Clinical routine use of dopamine transporter imaging in 516 consecutive patients. J Neurol. 2015;262(4):909–15. doi:10.1007/s00415-014-7634-y.
Booij J, Habraken JB, Bergmans P, Tissingh G, Winogrodzka A, Wolters EC, et al. Imaging of dopamine transporters with iodine-123-FP-CIT SPECT in healthy controls and patients with Parkinson’s disease. J Nucl Med : Off Publ Soc Nucl Med. 1998;39(11):1879–84.
Skanjeti A, Castellano G, Elia BO, Zotta M, Dazzara F, Manfredi M, et al. Multicenter Semiquantitative Evaluation of I-FP-CIT Brain SPECT. J Neuroimaging : Off J Am Soc Neuroimaging. 2015. doi:10.1111/jon.12242.
Tossici-Bolt L, Hoffmann SM, Kemp PM, Mehta RL, Fleming JS. Quantification of [123I]FP-CIT SPECT brain images: an accurate technique for measurement of the specific binding ratio. Eur J Nucl Med Mol Imaging. 2006;33(12):1491–9. doi:10.1007/s00259-006-0155-x.
Van Laere K, Casteels C, De Ceuninck L, Vanbilloen B, Maes A, Mortelmans L, et al. Dual-tracer dopamine transporter and perfusion SPECT in differential diagnosis of parkinsonism using template-based discriminant analysis. J Nucl Med : Off Publ Soc Nucl Med. 2006;47(3):384–92.
Kim JS, Cho H, Choi JY, Lee SH, Ryu YH, Lyoo CH, et al. Feasibility of computed tomography-guided methods for spatial normalization of dopamine transporter positron emission tomography image. PLoS One. 2015;10(7):e0132585. doi:10.1371/journal.pone.0132585.
Imabayashi E, Matsuda H, Tabira T, Arima K, Araki N, Ishii K, et al. Comparison between brain CT and MRI for voxel-based morphometry of Alzheimer’s disease. Brain Behav. 2013;3(4):487–93. doi:10.1002/brb3.146.
Sasaki K, Maikusa N, Imabayashi E, Yuasa T, Matsuda H. The feasibility of 11C-PIB-PET/CT for amyloid plaque burden: validation of the effectiveness of CT-based partial volume correction. Brain Behav. 2016. doi:10.1002/brb3.532.
Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38(1):95–113. doi:10.1016/j.neuroimage.2007.07.007.
Hammers A, Allom R, Koepp MJ, Free SL, Myers R, Lemieux L, et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;19(4):224–47. doi:10.1002/hbm.10123.
Varrone A, Dickson JC, Tossici-Bolt L, Sera T, Asenbaum S, Booij J, et al. European multicentre database of healthy controls for [123I]FP-CIT SPECT (ENC-DAT): age-related effects, gender differences and evaluation of different methods of analysis. Eur J Nucl Med Mol Imaging. 2013;40(2):213–27. doi:10.1007/s00259-012-2276-8.
Lapa C, Spehl TS, Brumberg J, Isaias IU, Schlogl S, Lassmann M, et al. Influence of CT-based attenuation correction on dopamine transporter SPECT with [(123)I]FP-CIT. Am J Nucl Med Mol Imaging. 2015;5(3):278–86.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by a grant from the Japan Foundation for Neuroscience and Mental Health and intramural research grant for neurological and psychiatric disorders of National Center of Neurology and Psychiatry (27-8).
Conflicts of interest
None.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
This retrospective study was approved by the institutional review board at the National Center of Neurology and Psychiatry Hospital, and the need for patient informed consent was waived.
Rights and permissions
About this article
Cite this article
Yokoyama, K., Imabayashi, E., Sumida, K. et al. Computed-tomography-guided anatomic standardization for quantitative assessment of dopamine transporter SPECT. Eur J Nucl Med Mol Imaging 44, 366–372 (2017). https://doi.org/10.1007/s00259-016-3496-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3496-0