Abstract
Implantable loop recorders (ILRs) are effective tools for detecting arrhythmias by long-term continuous heart rhythm monitoring. Benefits have been demonstrated even in pediatric patients. ILR with a long sensing vector has recently been designed to improve signal quality in terms of P wave visibility and R wave amplitude. However, there are no data on its use in pediatric patients. We considered a series of pediatric patients implanted with a long sensing vector ILR. Sensing performance, including R wave amplitude and P wave visibility, device-related complications, and diagnostic yield were collected. During follow-up, each patient guided by his/her parents/guardians was also asked to complete a brief questionnaire to assess patient acceptability of the device. Twenty-five consecutive pediatric patients (mean age 11.3 ± 3.5 years, 72% male) were enrolled. The insertion success rate was 100% on the first attempt with no complications. The median amplitude of the R wave was 1.15 mV (interquartile range, 1.01–1.42) with no significant differences between patients aged ≤ or > 10 years (p = 0.726) and between female and male (p = 0.483). P wave was classified as ‘always visible’ in 24/25 patients (96%). ILR was generally well accepted and tolerated by all involved patients. During a median follow-up of 297 days (117–317), we achieved in 5 patients a correlation between symptoms and rhythm disorders (20%) and ruled out significant arrhythmias in 6 symptomatic children (24%). Long sensing vector ILR showed to be well accepted, with good signal quality and an excellent safety profile even in pediatric patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Implantable loop recorders (ILRs) are subcutaneous devices used as diagnostic tools to detect heart rhythm disorders (brady or tachyarrhythmias) through long-term continuous heart rhythm monitoring. They are helpful in establishing a symptom–rhythm correlation in patients with recurrent and/or unexplained syncope, palpitations, cryptogenic stroke, or to record recurrences in patients undergoing catheter ablation for atrial fibrillation [1,2,3,4]. The reduction in device size and the simplification of the insertion procedure have facilitated their use even in the pediatric population; benefits from ILR implantation have been demonstrated in patients with or without congenital heart disease (CHD) or primary cardiac electrical disorders [5,6,7,8,9]. The ILR with long sensing vector is a novel device with a wider electrode spacing thanks to the addition of a long flexible antenna to the rigid case. It has been designed to overcome some issues of previous generation devices, as undersensing or noise artifacts, achieving convincing results in terms of signal quality, sensing parameters (P wave visibility and R wave amplitude), arrhythmia detection performance, and acceptability in the adult population [1,2,3,4]. No data are available on their use in the pediatric population.
The aim of this single-center, observational study was to assess the safety, device-related adverse events, sensing performance, and acceptability in a cohort of consecutive pediatric patients implanted with a long sensing vector ILR.
Materials and Methods
Study Design
We reviewed the medical records of all patients aged ≤ 16 years who underwent long sensing vector ILR implantation at the Pediatric and Congenital Heart Disease Electrophysiology Laboratory of our Cardiology Department (Monaldi Hospital, Naples, Italy) between October 2019 and August 2022. This study was conducted in accordance with the Declaration of Helsinki and the European directive for data protection (General Data Protection Regulation). Written informed consent was obtained from the patient’s parents/legal guardians in each case.
Baseline evaluation consisted of comprehensive clinical history, physical examination, 12 lead ECG, echocardiography, and 24 h ECG Holter monitoring in each patient. Indication for ILR implantation was provided according to the most recent literature and the latest guidelines. Clinical data were collected at the time of device implantation and included age, sex, height, weight, indication for ILR implantation and comorbidities. Further data on complications, detected arrhythmias, signal amplitudes, and patients’ acceptability were acquired by remote monitoring, outpatient clinical evaluation, and telephone contact (phone interviews).
Device Implantation
All patients were implanted with the BIOMONITOR III or BIOMONITOR IIIm (BIOTRONIK SE & Co. KG., Berlin, Germany), a recently introduced ILR with a peculiar combination of long sensing vector, miniaturized profile, and easy and fast insertion tool. The BIOMONITOR III is an ILR with a rigid case and a flexible silicone antenna that has been designed to increase the length of the sensing vector up to approximately 70 mm. The total volume and weight are 1.97 cm3 and 5 g, respectively, and the cross-sectional profile is 8.3 mm × 4.3 mm, similar to other commercially available novel devices, as the Confirm Rx (Abbott Medical, Plymouth, USA) (9.4 × 3.1 mm), the Lux-Dx (Boston Scientific, Massachusetts, USA) (7.2 × 4 mm) and the LinQ (Medtronic, Minneapolis, USA) (7 × 4 mm) (Fig. 1a). The device was implanted in two different insertion positions in this study: (1) parallel to the heart’s long axis, at the left parasternal region of the chest; (2) infraclavicular with the long flexible antenna directed to the heart (Fig. 1b). All procedures were performed in the cardiac catheterization laboratory. Insertion position was chosen based on of weight, chest dimensions and thickness of the subcutaneous tissue of each patient. Infraclavicular insertion was preferred in children with very thin subcutaneous tissue in the precordial area or in case of concerned parents/guardians about the esthetic result of the pectoral scar. All patients underwent deep sedation. Prophylactic one-single weight-based dose of cefazolin was administered intravenously in all patients prior to the procedure. The skin was prepared with topical chlorhexidine and local anesthetic (lidocaine). The skin incision and the injection of the device into the subcutaneous tissue were easily performed using the custom scalpel and the specific insertion tool provided by the manufacturer. We used wax coated braided silk or braided absorbable suture for the final skin suture, according to the operator’s discretion. All patients were discharged the day after the procedure.
Follow-Up
After ILR insertion, all patients received a CardioMessenger mobile device for remote monitoring (Home Monitoring®, BIOTRONIK SE & Co. KG., Berlin, Germany). All parents were instructed about the use. Devices were programmed for automatic transmission of events and subcutaneous electrocardiogram (sECG). Patients were scheduled to a programmed medical follow-up at our outpatient clinic at 1 week and at 6-month intervals. The mean R wave amplitude was automatically determined by the device each day. Remotely transmitted values in individual patients were averaged for the follow-up period. The presence of P waves was manually confirmed on a printout of periodic sECG transmissions and sECG tracings of clinically relevant episodes by independent electrophysiologists as ‘always visible,’ ‘intermittently visible’ (i.e., not visible in all available beats), or ‘never visible’. The noise burden, defined as the percentage of time during which the patient's rhythm cannot be assessed due to artifacts, was also collected from device diagnostics. Finally, a survey was conducted to assess the acceptability of the device by patients and parents for both physical and psychological components.
Data Analysis
Data are presented as mean ± standard deviation or, if not normally distributed, as median (interquartile range [IQR]) for continuous variables and percentages for dichotomous variables. The R wave amplitude was compared between subgroups with the Mann–Whitney test. Statistical analysis was performed using STATA (version 17.0, StataCorp LP, College Station, TX, USA). Statistical significance was achieved with a p-value of < 0.05.
Results
Patient Characteristics
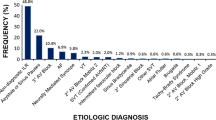
Twenty-five consecutive pediatric patients were included and completed the study follow-up. Baseline characteristics of the study cohort are shown in Table 1. The mean age was 11.3 ± 3.5 years (range 5–16). Eleven patients (44%) were ≤ 10 years old and 18 (72%) were male. The most frequent indication for ILR implantation was unexplained recurrent syncope (n = 14, 56%), followed by risk stratification for channelopathies (n = 5, 20%) and palpitations (n = 3, 12%). Most of patients had no other comorbidities (n = 9, 36%). A family history of sudden cardiac death (SCD) was present in 20% of cases (n = 5). Four patients had a diagnosis of CHD (16%). The success rate of the insertion was 100% on the first attempt with no periprocedural complications. Nineteen devices were inserted in a parallel position to the heart’s long axis (76%). The remaining 6 devices were inserted in an infraclavicular position (24%).
Signal Analysis
Signal amplitude and noise burden analysis were performed in all enrolled patients. At device interrogation, the median R wave amplitude was 1.15 mV (IQR, 1.01–1.42 mV). Furthermore, the R wave amplitude was similar between patients aged ≤ or > 10 years old (1.10 [1.00–1.36] vs. 1.18 [1.01–1.57], p = 0.726) and between female and male (1.18 [0.6–1.42] vs. 1.13 [1.01–1.57], p = 0.483). P wave was classified as ‘always visible’ on all periodic sECGs in 24/25 patients (96%). The visibility of the P wave was intermittent only in one case. The median noise burden retrieved from device diagnostics was 0.5% (0–1.3%).
Device Acceptability
At the end of the study follow-up, all children guided by their parents/guardians completed an evaluation questionnaire (Table 2). The implanted device resulted very well tolerated in almost all patients. Pain, skin irritation, or movement restrictions were described as absent (score 1) in > 70% of the children and were always ranked between 1 and 3. We also asked their parents the impact of ILR implantation on their daily life. The ILR was not perceived as intrusive to privacy and was not of concern in almost all cases. On the contrary, parents reported a high feeling of safety for their children due to device implantation and continuous monitoring of heart rhythm (90% of cases).
Follow-Up
During a median follow-up of 297 days (IQR 117–317), no device-related complications or serious adverse events occurred in all children. The ILR position was anatomically stable in all patients during follow-up. Thanks to remote monitoring and automatic transmission, we achieved a correlation between symptoms and rhythm disorders in 5 patients (20%) (Fig. 2). One patient with unexplained palpitations had a diagnosis of paroxysmal supraventricular tachycardia (PSVT). Another one who complained of palpitations showed brief episodes of ectopic atrial tachycardia (EAT) during symptoms (Fig. 3a). In 3 children with unexplained syncope, 2 patients had syncope episodes with asystole (maximum recorded RR interval 3.2 s), while 1 patient had a diagnosis of PSVT. A patient with PSVT started medical therapy (sotalol) and was listed for an electrophysiologic (EP) study. The other one underwent EP study and radiofrequency catheter ablation of atrioventricular nodal reentrant tachycardia. Both children with asystole received a clinical diagnosis of reflex cardioinhibitory syncope, so no further therapeutic interventions were required. Six more patients (24%) implanted for syncope episodes (n = 3), recurrent palpitations (n = 1), BrS (n = 1), and LQTS (n = 1) had symptoms during follow-up (near syncope, n = 1; dizziness, n = 2; palpitations, n = 3) but no significant arrhythmias were detected (Fig. 3b). ILR was implanted in 5 patients (20%) with inherited arrhythmia syndromes (BrS and LQTS) in our cohort (Fig. 4). No malignant arrhythmias were observed during follow-up in this high-risk group of children.
a Paroxysmal supraventricular tachycardia in a child suffering from palpitations. Atrioventricular nodal reentrant tachycardia (AVNRT) was suspected due to regular and narrow QRS complexes with abrupt onset and offset. Electrophysiologic study confirmed the diagnosis of AVNRT. b Cardioinhibitory syncope with sinus bradycardia and transient junctional rhythm. Paroxysmal atrioventricular block was excluded thanks to a good P wave visibility
Discussion
Indications for ILR in Pediatric Patients
Unexplained recurrent syncope was the most common indication for ILR implantation (56%) in this study. According to the latest guidelines, ILR is recommended in high-risk patients with unexplained syncope, without indication for pacemaker or ICD, and is reasonable in patients with recurrent syncope of uncertain origin, but without a high risk of SCD [9]. ILR implantation should be considered when arrhythmogenic syncope is suspected but not proven [9, 10].
Other indications were palpitations with non-diagnostic non-invasive evaluation and documented episodes of brady and tachyarrhythmias with unclear symptoms. In our series, two children (8%) underwent ILR implant due to unclear symptoms (dizziness, near syncope) and previously documented episodes of bradyarrhythmia. ILR may be useful in the monitoring of bradyarrhythmias and their correlation with clinical symptoms or in patients at risk for intermittent or progressive atrioventricular (AV) block [9]. A patient with corrected CHD, unclear symptoms and non-sustained ventricular tachycardia (nsVT) in our series was implanted to exclude episodes of sustained VT, as ILR may identify subclinical arrhythmias that may warrant changes in patient management, especially in children with cardiac diseases associated with increased arrhythmic risk [9].
ILR was implanted in 5 patients (20%) with inherited arrhythmia syndromes (BrS and LQTS) in our cohort [9]. According to the latest Italian recommendations [11], ILR was implanted in asymptomatic patients with BrS and high-risk family history (ICD implantation, SCD) and in symptomatic patients not eligible for ICD implantation due to unclear symptoms (e.g., history of palpitations or lipothymia). ILR was also implanted in children with a diagnosis of LQTS and palpitations to rule out uncovered ventricular arrhythmias [9,10,11] (Fig. 4). In our series, no arrhythmic events were observed after ILR implant during follow-up in this high-risk group of patients. It confirms that symptoms are often not a reliable marker of arrhythmias in pediatric patients, and this is even more important in children with inherited arrhythmia syndromes because symptoms are included in risk stratification algorithms [7]. ILR and automatic transmission may be considered to uncover subclinical arrhythmias when caring for these little patients to guide clinical decision-making, regarding the start of therapy, titrating activity levels, addition of medications, or indication to ICD implantation, also providing a psychological support to parents or guardians [7, 11,12,13].
Device Performance in Adults and Pediatric Patients
The BIOMONITOR III ILR represents a novel device for prolonged heart rhythm monitoring with smaller hardware, greater longevity, and improved sensing algorithms compared to previous generation devices [1,2,3,4]. First-generation devices were burdened with problems such as device dimensions, artifacts, and low diagnostic power. The BIOMONITOR III demonstrated high diagnostic accuracy and sensing performance with low noise burden in the adult population [1,2,3,4]. The implantation technique was found to be safe and fast with a low complication rate (e.g., wound infection, device displacement or protrusion, post-implant pain and/or discomfort). Acceptability was high for the overwhelming majority of patients.
To the best of our knowledge, this study represents the only study to date investigating the safety and acceptability of the long sensing vector ILR (BIOMONITOR III) in pediatric patients. The primary aim was to investigate the safety of device implantation in this population. In our cohort, insertion procedure was safe and easy, irrespective of the patient characteristics or the implant position. No children experienced acute complications or adverse events during follow-up. The secondary aim was to evaluate the sensing performance and acceptability. We confirmed the high signal quality of this device in terms of R wave amplitude and noise burden also in pediatric patients. We achieved a high mean R wave amplitude (1.15 mV [1.01–1.42 mV]) with a good minimum measured value (0.5 mV). The size of the R wave can change in children over time, due to growth in weight and height and changes in body habitus. We did not observe significant changes on the R wave in amplitude and morphology during the follow-up period. However, the median follow-up period was less than one year in this study, and it could have affected our findings. Further studies with longer follow-up are warranted to confirm our preliminary results.
Considering the greater mobility and restlessness of pediatric patients in daily activity compared to adults (e.g., physical activities at school, sport, games), the median noise burden we found was very low (0.5%), with no patient exceeding 5%. The capability to detect P waves is of high clinical relevance in establishing the type of arrhythmia. P waves could be identified in all patients in our study, with stable and constant P wave detection in 24/25 children (96%). Furthermore, neither noise burden nor P and R wave sensing appeared to be influenced by patient characteristics.
Our pediatric study cohort demonstrated convincing results in view of previous reports that evaluated the sensing performance of the BIOMONITOR III in adults. Deneke et al. [1] reported a mean R wave amplitude of 0.73 ± 0.4 mV with a gross P wave visibility of 95.1% and a median noise burden < 10 min/day in a large adult population (653 adult patients followed for a median of 274 days). Reinsch et al. [2] obtained a mean R wave amplitude of 0.81 ± 0.39 mV with a median noise burden < 2% after a mean follow-up of 107 ± 59 days. Mariani et al. [3] reported a mean R wave amplitude of 0.7 ± 0.37 mV with a median noise burden of 0.19% and good P wave visibility (89 ± 24%) after a mean follow-up period of 35.2 ± 18.5 days. Furthermore, our results appear to be satisfactory in light of recent reports in the literature evaluating the performance of other currently available next-generation ILRs in pediatric patients [14,15,16].
In the present study, records obtained from automatic transmissions and remote monitoring after ILR implantation were useful to achieve a diagnosis in 5 symptomatic children (20%) and to rule out arrhythmias in 6 symptomatic patients (24%). No significant arrhythmias were detected among the other children. Thanks to the ILR, three patients achieved diagnosis of SVT after negative conventional diagnostic work-up: sporadic or non-sustained episodes of SVT in children sometimes may result to be uncovered by non-invasive diagnostic testing, such as prolonged Holter monitoring. The increased P wave visibility and R wave amplitude can be useful to discriminate the type of arrhythmia, evaluating P wave morphology, RP interval, narrow or wide QRS complexes, also guiding the medical management. In two children with undiagnosed syncope episodes and inconclusive non-invasive tests, including inconclusive head-up tilt test, the increased P wave visibility represented a significant advantage to confirm the clinical suspect of reflex syncope: a typical cardioinhibitory response with sinus pauses and junctional rhythm was observed during the clinical episode on automatic sECG transmissions, excluding paroxysmal AV block.
The patients’ and parents’ acceptability of the BIOMONITOR III resulted excellent. None of the patients reported significant device-related problems or discomfort. With regard to psychological components, no significant worries about esthetics or interference with social life, sports, or education were found. Most parents were not concerned after device implantation and felt safer because their children were constantly monitored.
Remote Monitoring
In our experience, remote monitoring allowed us to follow-up our little patients, avoiding unnecessary in-person visits and in-hospital device interrogations, by monitoring diagnostics remotely, especially during the COVID-19 outbreak. It was useful in communicating effectively with their families by phone contact or email, reassuring parents or guardians about the health of their children. We suppose that it played an important role in the high sense of safety that resulted from our questionnaires. Despite clinical benefits, remote monitoring requires an increased work overload to physicians, nurses, and hospital stuff to check regularly automatic transmissions and to communicate with families during follow-up if symptoms occur or arrhythmias are detected [17,18,19]. More studies are required to better assess the impact of remote monitoring on the early detection of arrhythmias and the symptom–rhythm correlation in the pediatric population.
Limitations
Limitations of this study include the retrospective and single-center design with a small number of patients and a heterogeneous population. A low number of significant arrhythmic events were encountered in our study, so detection performance needs to be re-assessed after a larger number of arrhythmia episodes. The lowest age used for this device was 5 years and lowest weight was 23 kg in this study. Therefore, our results could not be generalizable to the entire pediatric population, especially for very small children. The influence of device insertion position on sensing performance and noise burden was not evaluated in view of the small population. In our experience, it appeared to be negligible, but larger investigations are required to find the best position for this new ILR in this setting. Further studies are warranted to compare current generation ILRs with different vector length in the pediatric population and to better define the benefit of long sensing vector ILR implantation.
Conclusion
Based on our preliminary experience, the use of a long sensing vector ILR (BIOMONITOR III) was well accepted, with a good signal quality and excellent safety profile in a cohort of pediatric patients. The amplitude of the R wave was good regardless of age or gender, with good P wave visibility and a very low noise burden, confirming the excellent sensing performance demonstrated in the adult population.
References
Deneke T, Cabanas P, Hofer D, Gaspar T, Pierre B, Bisignani G, Pathak RK, Sanfins VM, Martens E, Mansourati J, Berruezo-Sanchez A, Wiemer M, Hain A, Pezawas T, Wenzel B, Lau D (2022) BIO|MASTER.BIOMONITOR III study and BIO|STREAM.ICM Rregistry investigators: New-generation miniaturized insertable cardiac monitor with a long sensing vector: Insertion procedure, sensing performance, and home monitoring transmission success in a real-world population. Heart Rhythm O2 3(2):152–159. https://doi.org/10.1016/j.hroo.2022.01.010
Reinsch N, Füting A, Höwel D, Neven K (2022) The BIOMONITOR III injectable cardiac monitor: clinical experience with a novel injectable cardiac monitor. J Clin Med 11(6):1634. https://doi.org/10.3390/jcm11061634
Mariani JA, Weerasooriya R, van den Brink O, Mohamed U, Gould PA, Pathak RK, Lin T, Conradie A, Illes P, Pavia S, Rajamani K, Lovibond S, Matthews I, Difiore D, Arumugam D, Schrader J, Lau DH (2020) Miniaturized implantable cardiac monitor with a long sensing vector (BIOMONITOR III): insertion procedure assessment, sensing performance, and home monitoring transmission success. J Electrocardiol 60:118–125. https://doi.org/10.1016/j.jelectrocard.2020.04.004
Forleo GB, Amellone C, Sacchi R, Lombardi L, Lucciola MT, Scotti V, Viecca M, Schiavone M, Giacopelli D, Giammaria M (2021) Factors affecting signal quality in implantable cardiac monitors with long sensing vector. J Arrhythm 37(4):1061–1068. https://doi.org/10.1002/joa3.12585
Rossano J, Bloemers B, Sreeram N, Balaji S, Shah MJ (2003) Efficacy of implantable loop recorders in establishing symptom-rhythm correlation in young patients with syncope and palpitations. Pediatrics 112(3 Pt 1):e228–e233. https://doi.org/10.1542/peds.112.3.e228
Al Dhahri KN, Potts JE, Chiu CC, Hamilton RM, Sanatani S (2009) Are implantable loop recorders useful in detecting arrhythmias in children with unexplained syncope? Pacing Clin Electrophysiol 32(11):1422–1427. https://doi.org/10.1111/j.1540-8159.2009.02486.x
Avari Silva JN, Bromberg BI, Emge FK, Bowman TM, Van Hare GF (2016) Implantable Loop Recorder Monitoring for Refining Management of Children With Inherited Arrhythmia Syndromes. J Am Heart Assoc 5(6):e003632. https://doi.org/10.1161/JAHA.116.003632
Gass M, Apitz C, Salehi-Gilani S, Ziemer G, Hofbeck M (2006) Use of the implantable loop recorder in children and adolescents. Cardiol Young 16(6):572–578. https://doi.org/10.1017/S1047951106001156
Shah MJ, Silka MJ, Silva JNA, Balaji S, Beach CM, Benjamin MN, Berul CI, Cannon B, Cecchin F, Cohen MI, Dalal AS, Dechert BE, Foster A, Gebauer R, Gonzalez Corcia MC, Kannankeril PJ, Karpawich PP, Kim JJ, Krishna MR, Kubuš P, LaPage MJ, Mah DY, Malloy-Walton L, Miyazaki A, Motonaga KS, Niu MC, Olen M, Paul T, Rosenthal E, Saarel EV, Silvetti MS, Stephenson EA, Tan RB, Triedman J, Von Bergen NH, Wackel PL (2021) Document Reviewers: Philip M. Chang, Fabrizio Drago, Anne M. Dubin, Susan P. Etheridge, Apichai Kongpatanayothin, Jose Manuel Moltedo, Ashish A. Nabar and George F. Van Hare. 2021 PACES expert consensus statement on the indications and management of cardiovascular implantable electronic devices in pediatric patients. Cardiol Young 31(11):1738–1769. https://doi.org/10.1017/S1047951121003413
Babikar A, Hynes B, Ward N, Oslizok P, Walsh K, Keane D (2008) A retrospective study of the clinical experience of the implantable loop recorder in a paediatric setting. Int J Clin Pract 62(10):1520–1525. https://doi.org/10.1111/j.1742-1241.2007.01389.x
Drago F, Bloise R, Bronzetti G, Leoni L, Porcedda G, Sarubbi B, Defilippo P, Gulletta S, Scaglione M (2020) Italian Association of Arrhythmology and Cardiac Pacing (AIAC) and the Italian Society of Pediatric Cardiology (SICP). Italian recommendations for the management of pediatric patients under twelve years of age with suspected or manifest Brugada syndrome. Minerva Pediatr 72(1):1–13. https://doi.org/10.23736/S0026-4946.19.05759-1
Sarubbi B, Colonna D, Correra A, Romeo E, D’Alto M, Palladino MT, Virno S, D’Onofrio A, Russo MG (2022) Subcutaneous implantable cardioverter defibrillator in children and adolescents: results from the S-ICD “Monaldi care” registry. J Interv Card Electrophysiol 63(2):283–293. https://doi.org/10.1007/s10840-021-00966-4
Sarubbi B, Farina G, Colonna D, Correra A, Romeo E, Ciriello GD, Oppido G, Russo MG (2022) Subcutaneous finger cardioverter-defibrillator in low weight paediatric patients: a case series. Monaldi Arch Chest Dis. https://doi.org/10.4081/monaldi.2022.2203
Yoon JG, Fares M, Hoyt W Jr, Snyder CS (2021) Diagnostic accuracy and safety of confirm Rx™ insertable cardiac monitor in pediatric patients. Pediatr Cardiol 42(1):142–147. https://doi.org/10.1007/s00246-020-02463-3
D’Souza R, Thomas E, Macicek S, Aziz P, Shivapour JK, Snyder C (2017) P- and R-wave amplitude sensed by reveal LINQ™ loop recorder in pediatric patients. J Innov Card Rhythm Manag 8(1):2584–2588. https://doi.org/10.19102/icrm.2017.080102
Bezzerides VJ, Walsh A, Martuscello M, Escudero CA, Gauvreau K, Lam G, Abrams DJ, Triedman JK, Alexander ME, Bevilacqua L, Mah DY (2019) The real-world utility of the LINQ implantable loop recorder in pediatric and adult congenital heart patients. JACC Clin Electrophysiol 5(2):245–251. https://doi.org/10.1016/j.jacep.2018.09.016
Lorimer D Jr, Dalal AS, Miller N, Roelle L, Orr WB, Van Hare GF, Avari Silva JN (2021) Comparing patient and family usability of insertable cardiac monitors in a pediatric cohort: Patient external activator versus smartphone transmission. Heart Rhythm O2 2(2):201–204. https://doi.org/10.1016/j.hroo.2021.03.004
Russo V, Rapacciuolo A, Rago A, Tavoletta V, De Vivo S, Ammirati G, Pergola V, Ciriello GD, Napoli P, Nigro G, D’Onofrio A (2022) Early evaluation of atrial high rate episodes using remote monitoring in pacemaker patients: results from the RAPID study. J Arrhythm 38(2):213–220. https://doi.org/10.1002/joa3.12685
De Coster M, Demolder A, De Meyer V, Vandenbulcke F, Van Heuverswyn F, De Pooter J (2020) Diagnostic accuracy of R-wave detection by insertable cardiac monitors. Pacing Clin Electrophysiol 43(5):511–517. https://doi.org/10.1111/pace.13912
Acknowledgements
Special thanks to the Pediatric and Adult Congenital Heart Disease Unit nursing staff and specially to the head nurses Mrs. Assunta Carandente, Mrs Monica Iacona, and Mrs Annunziata Orefice for their essential contribution and support in maintaining high-quality standard of care for our patients. We thank our data manager Dr. Gabriella Piccolo, Dr. Nadia Puzone, Dr. Cecilia Spinelli Barrile, and Dr. Tiziana Varriale for data collecting and analysis and support in remote control assistance. We thank furthermore Eng. Andrea Spadaro Guerra and Eng. Daniele Giacopelli for their support in data analysis.
Funding
No funding sources have been given for this study.
Author information
Authors and Affiliations
Contributions
GDC and BS contributed to write, review, and image editing. NG, GP, and NB contributed to data collection and review. AC, DC, and ER contributed to production of tables/figures and review. MDM, RE, VR, and MGR contributed to review. All authors approved the manuscript text.
Corresponding author
Ethics declarations
Conflict of interest
All authors report no relationship that could be construed as a conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ciriello, G.D., Grimaldi, N., Papaccioli, G. et al. Implantable Loop Recorder with Long Sensing Vector: Safety, Acceptability, and Sensing Performance in Pediatric Patients. Pediatr Cardiol 44, 1068–1075 (2023). https://doi.org/10.1007/s00246-022-03082-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-022-03082-w