Abstract
The intracellular location of polycystin-2 is a hotly debated topic in the field of polycystic kidney disease. Two not necessarily mutually exclusive hypotheses state that polycystin-2 is located in the endoplasmic reticulum or in the plasma membrane, respectively. Although a variety of techniques have been employed to prove one or the other location, no definite consensus has been reached yet. It is generally acknowledged, however, that the COOH-terminus of polycystin-2 contains a retention signal for the endoplasmic reticulum. Another facet has been added to the discussion due to the fact that many genes mutated in patients with cystic kidney diseases, among them PKD2, encode proteins which have been detected in primary cilia. Since there is no evidence that the endoplasmic reticulum extends into the primary cilium, polycystin-2 has to reach the plasma membrane at least in this case. An unbiased approach towards elucidating the physiological location of polycystin-2 would involve the characterization of its intracellular trafficking. Using the COOH-terminus of polycystin-2 in a two-hybrid screen, my group has identified a novel coiled-coil protein which we call PIGEA-14 (polycystin-2 interactor, Golgi- and endoplasmic reticulum-associated protein with a molecular weight of 14 kDa). PIGEA-14 also interacts with GM130, a protein associated with the Golgi matrix, and may therefore represent one important component of the trafficking machinery for polycystin-2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Polycystin-2 is a member of the Trp family of cation channels
Polycystic kidney disease is one of the most common monogenetic diseases, its prevalence has been estimated to be at least one in 1,000. It is characterized by the lifelong expansion of renal tubules, although symptoms affecting the liver, the pancreas and the cardiovascular system are also seen on a regular basis (for a recent review see Wilson 2004). A number of hypotheses have been advanced to explain cyst formation and cyst growth, such as a change in cell polarity (Wilson 1997), an altered matrix composition (Calvet 1993) or a dysbalance between cell proliferation and apoptosis (Gallagher et al. 2002), but the mechanisms had to remain speculative until the identification of the mutated genes.
The positional cloning of the PKD1 (European Polycystic Kidney Disease Consortium 1994) and PKD2 (Mochizuki et al. 1996) genes raised great hopes for a better understanding of the pathogenetic events occurring in patients with autosomal-dominant polycystic kidney disease. The PKD2 gene codes for polycystin-2, a 968-amino acid protein with six putative transmembrane domains, whose NH2- and COOH-termini are predicted to extend into the cytoplasm. Together with three related proteins, which are called polycystin-2L, polycystin-2L2 and mucolipin, these four proteins form a separate branch of the large family of Trp (transient receptor potential) proteins, a novel class of cation channels (Montell et al. 2002). Due to their sequence homology with cation channels, experiments were soon initiated to examine the ion-conducting properties of these proteins. For polycystin-2 (González-Perrett et al. 2001; Hanaoka et al. 2000; Koulen et al. 2002; Luo et al. 2003; Vassilev et al. 2001), polycystin-2L (Chen et al. 1999) and mucolipin (Raychowdhury et al. 2004) cation channel activity indeed has been demonstrated, whereas no reports have been published yet whether polycystin-2L2 can conduct cations.
The exclusive intracellular location of polycystin-2 remains controversial
A lot of evidence for the location of polycystin-2 in the endoplasmic reticulum has been provided by the group of Stefan Somlo. Using immunocytochemistry his group was able to demonstrate a fine reticular distribution of exogenous polycystin-2 in HEK 293, MDCK, LLC-PK1 and HeLa cells, which overlapped with that of marker proteins of the endoplasmic reticulum (Cai et al. 1999; Koulen et al. 2002). The immunocytochemical results were corroborated by biochemical experiments. Polycystin-2 contains several potential N-glycosylation sites and indeed it has been shown to be N-glycosylated (Cai et al. 1999; Hidaka et al. 2004). Again employing HEK 293 cells, it was demonstrated that polycystin-2 is still sensitive to treatment with endoglycosidase H (Cai et al. 1999), an enzyme which usually removes N-linked sugar moieties only if the protein has not progressed beyond the cis-compartment of the Golgi apparatus. By density gradient centrifugation of HEK 293 homogenates, polycystin-2 was found to co-migrate with a marker of the endoplasmic reticulum (Cai et al. 1999), and surface biotinylation of LLC-PK1 cells demonstrated no evidence of full-length polycystin-2 at the cell surface (Cai et al. 1999). Obviously the kidney is the appropriate tissue to determine the exact location of polycystin-2, and both in human kidney using sensitivity to endoglycosidase H (Cai et al. 1999) and in murine kidney using density gradient centrifugation and again sensitivity to endoglycosidase H (Koulen et al. 2002) polycystin-2 was found in the endoplasmic reticulum. Our own investigations in LLC-PK1 cells and a number of other cell lines (Gallagher et al. 2000; Hidaka et al. 2004) have confirmed the above findings, but there is conflicting evidence from other groups.
Whereas an exogenous polycystin-2 protein was detected in intracellular membranes of MDCK (Scheffers et al. 2002) and mIMCD3 cells (Luo et al. 2003) by immunocytochemistry, the endogenous polycystin-2 protein was found both in intracellular membranes and in the plasma membrane (in particular cell–cell contacts) of MDCK and mIMCD3 cells by immunocytochemistry (Luo et al. 2003; Scheffers et al. 2002), density gradient centrifugation (Scheffers et al. 2002) and surface biotinylation (Luo et al. 2003). In LLC-PK1 cells, however, a predominant or even exclusive location of polycystin-2 in intracellular membranes was detected by immunocytochemistry (Luo et al. 2003; Scheffers et al. 2002). These data suggest that the cellular environment, i.e. the proteome of a given cell, influences the subcellular location of polycystin-2. Evidence for such a hypothesis comes from experiments conducted in CHO cells. It was shown by immunocytochemistry that in the absence of polycystin-1, the protein encoded by the PKD1 gene, polycystin-2 is located in intracellular membranes of CHO cells, whereas in its presence polycystin-2 reached the plasma membrane (Hanaoka et al. 2000). Since polycystin-1 and polycystin-2 interact through their respective COOH-termini (Qian et al. 1997; Tsiokas et al. 1997), polycystin-1 may mask the retention signal of polycystin-2 for the endoplasmic reticulum by direct interaction or by influencing the folding of polycystin-2. However, results obtained with cell lines established from transgenic mice with a human PKD1 gene indicate that the situation is not so simple. Because of the presence of the human polycystin-1 protein the prediction would have been that the murine polycystin-2 protein is predominantly present in the plasma membrane, which is not the case. By immunocytochemistry and density gradient centrifugation the majority of polycystin-2 was detected in the endoplasmic reticulum of these cell lines (by the latter technique this was also demonstrated for human kidney), but some of the protein also seemed to be present in the plasma membrane. Somewhat surprisingly, all of polycystin-2 in the cell lines and in human kidney was sensitive to a treatment with endoglycosidase H (Newby et al. 2002).
How do proteins reach the primary cilium?
After having been neglected for a long time, primary cilia have experienced a renaissance due to the location of many cystic kidney disease-associated proteins in this organelle (Pazour 2004). The function of primary cilia is still a matter of debate, but they are thought to play a mechanosensory role (Nauli et al. 2003; Pazour 2004; Praetorius and Spring 2003). Polycystin-2 also has been localized to cilia (Pazour et al. 2002; Yoder et al. 2002), and an interesting question concerns the routing of this and other integral membrane proteins to the cilium. Since no endoplasmic reticulum has been detected in primary cilia yet, at least in this location polycystin-2 has to reach the plasma membrane. Due to the fact that the primary cilium only represents a small portion of the plasma membrane, it may be well possible that biotinylated polycystin-2 was not detected because of its low abundance in the plasma membrane.
Due to its amenability to genetic and biochemical manipulation, the green alga Chlamydomonas has served as the model organism to study intraflagellar transport, whereas only very little is known about primary cilia in mammalian cells (Cole 2003; Pazour 2004). In particular, it is not clear how membrane proteins reach the ciliary membrane. Although it is generally believed that no vesicles are transported within cilia and flagella (Kozminski et al. 1993), other evidence has been presented as well (Jensen et al. 2004). Since some membrane proteins are concentrated in the cilium, something like a barrier has to exist at the base of the cilium to prevent diffusion out of the cilium, which could be the “ciliary necklace” (Gilula and Satir 1972). If integral membrane proteins are transported to the apical plasma membrane and then reach the primary cilium by diffusion, the barrier at the base of the cilium would have to be a “one-way street” in so far as the integral membrane proteins would be able to enter the primary cilium through the barrier but not leave it. Such a scenario may be unlikely, it is rather conceivable that the base of the cilium serves as a docking station for transport vesicles (Fig. 1), for which there is some evidence (Rogers et al. 2004). Alternatively, the integral membrane proteins may be anchored to the underlying cytoskeleton similar to what has been described for the Drosophila photoreceptor cells (Montell 1998).
Models for the trafficking of ciliary proteins. There are essentially three possibilities how proteins may reach the primary cilium and are retained there. Non-integral membrane proteins may go to the basal body and move up and down the cilium via intraflagellar transport (IFT). Since there is some controversy about the presence of vesicles inside of primary cilia, integral membrane proteins may have to reach the primary cilium through a different route. This could either be through the adjacent apical plasma membrane (depicted on the right) or by docking to a so far hypothetical structure (possibly the ciliary necklace) at the base of the cilium which at the same time may serve as a barrier against the adjacent membrane (depicted on the left)
Polycystin-2 interacts with PIGEA-14
Isolation of PIGEA-14
The COOH-terminus of polycystin-2 is an important determinant for its subcellular location, and we therefore used this part of the protein in a yeast two-hybrid screen to isolate interacting proteins. Several of the clones isolated encoded a novel protein which we call PIGEA-14. The human PIGEA-14 protein is a 126-amino acid protein with a predicted molecular mass of 14.2 kDa, orthologous proteins were found in other vertebrate species but neither in Drosophila melanogaster nor in Caenorhabditis elegans (Fig. 2a). Using structural prediction programs, only a coiled-coil motif was detected in PIGEA-14 (Fig. 2b), and its initial biochemical characterization shows that it is not an integral membrane protein (Hidaka et al. 2004). Since both PIGEA-14 and polycystin-2 contain a coiled-coil motif it was tempting to speculate that the interaction between the two proteins is mediated by these domains. However, only the coiled-coil motif of PIGEA-14 is necessary for the association with polycystin-2, whereas the corresponding region in polycystin-2 lies downstream of the coiled-coil motif (Hidaka et al. 2004). The specificity of the interaction between PIGEA-14 and polycystin-2 was demonstrated by testing for interaction against other proteins with coiled-coil motifs and by co-precipitation. Neither PIGEA-14 nor polycystin-2 interacted with the leucine zipper domains of c-Fos and c-Jun, furthermore PIGEA-14 also did not interact with the closely related polycystin-2L protein (Hidaka et al. 2004). In order to demonstrate that both proteins not only interacted in yeast, but also in mammalian cells, epitope-tagged versions of either protein were introduced into LLC-PK1 cells, a porcine kidney epithelial cell line. PIGEA-14 co-precipitated very robustly with polycystin-2 and vice versa, thus emphasizing the strong association of the two proteins (Hidaka et al. 2004).
PIGEA-14, a novel coiled-coil protein interacting with polycystin-2. a Sequence comparison between the PIGEA-14 proteins from different species. The bar above the sequence indicates the coiled-coil motif. b Helical wheel presentation of the coiled-coil motif of PIGEA-14. Taken with permission from Hidaka et al. (2004)
PIGEA-14 interacts with GM130 and affects the intracellular distribution of polycystin-2
Because the primary sequence of PIGEA-14 provided no clue for its function, we performed another two-hybrid screen, only this time using PIGEA-14 as a bait. Among the candidate proteins identified were PIGEA-14 itself and GM130, a component of the Golgi matrix (Hidaka et al. 2004). As already shown for the association between PIGEA-14 and polycystin-2, both for the self-interaction of PIGEA-14 and for the interaction of PIGEA-14 with GM130 the coiled-coil motif of PIGEA-14 was necessary.
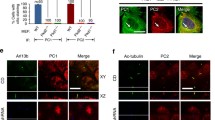
GM130 not only is a component of the Golgi matrix, but it also appears to be involved in the trafficking of vesicles from the endoplasmic reticulum to the Golgi apparatus (Barr and Short 2003; Gillingham and Munro 2003). We therefore next asked the question how the intracellular distribution of polycystin-2 was affected by PIGEA-14. Similar to what we had described before for polycystin-2 (Gallagher et al. 2000), PIGEA-14 was detected in a reticular distribution in the cytoplasm of HeLa and LLC-PK1 cells in the absence of polycystin-2 (Fig. 3a). When both proteins were present, however, a “patchy” distribution was observed for either of them (Fig. 3b). Using various controls we were able to demonstrate that this striking redistribution depended on the physical interaction between PIGEA-14 and polycystin-2 and was not simply due to the overexpression of both proteins (Hidaka et al. 2004), furthermore the “patches” also contained a lumen (Fig. 3b). These vesicular organelles did not contain GM130 or other proteins of the Golgi apparatus (Hidaka et al. 2004), as a matter of fact only TGN46, a protein associated with the trans-Golgi network, was also present among all the proteins tested (Fig. 3c). In a fashion similar to that of PIGEA-14 and polycystin-2, TGN46 changed its distribution when both PIGEA-14 and polycystin-2 were produced in HeLa and LLC-PK1 cells. In contrast to polycystin-2, however, PIGEA-14 was not detectable in cilia under any circumstances (Hidaka et al. 2004). The presence of polycystin-2 in a TGN46-positive compartment upon the presence of PIGEA-14 made us predict that polycystin-2 should have moved through the Golgi apparatus and became resistant to a digest with endoglycosidase H. Much to our surprise this was not the case (Fig. 3d).
Intracellular distribution of PIGEA-14 and polycystin-2 in HeLa cells. a In the absence of the other protein, polycystin-2 and PIGEA-14 are distributed in a reticular pattern in the cytoplasm of stably transfected HeLa cells. b When both proteins are produced together in HeLa cells, they are concentrated in vesicular organelles (inset shows higher magnification). c These vesicular organelles are also stained with an antibody against TGN46, a marker of the trans-Golgi network. d Both in the absence and in the presence of PIGEA-14, polycystin-2 is still completely sensitive to the action of endoglycosidase H. Taken with permission from Hidaka et al. (2004)
Conclusions and perspectives
The identification of PIGEA-14 has begun to shed the first light on the mechanisms underlying the intracellular trafficking of polycystin-2. It is undisputed that polycystin-2 contains a retention signal for the endoplasmic reticulum in its COOH-terminus, which does not belong to any of the known retention signals. The fact that this region in polycystin-2 overlaps with the domain mediating the interaction with PIGEA-14 fits well with our observation that the production of PIGEA-14 results in the redistribution of polycystin-2 into a TGN46-positive compartment. However, it remains to be shown that the TGN46-positive organelle really is the trans-Golgi network or an as yet unidentified intracellular compartment. The fact that polycystin-2 in the TGN46-positive compartment is still sensitive to a digest with endoglycosidase H argues against the trans-Golgi network, although it has to be kept in mind that not all glycoproteins which have passed through the Golgi apparatus become resistant to the action of endoglycosidase H. As has been mentioned before, studies performed with human kidney tissue have shown that by density gradient centrifugation polycystin-2 is present in the endoplasmic reticulum and in the plasma membrane, but that all of polycystin-2 is sensitive to a digest with endoglycosidase H (Newby et al. 2002). Since a truncated polycystin-2 protein lacking the COOH-terminus reaches the plasma membrane and becomes resistant to the action of endoglycosidase H this may suggest that the full-length polycystin-2 protein does not migrate through the Golgi apparatus. Why does polycystin-2 get stuck in a TGN46-positive compartment and does not move on to the plasma membrane? How does it reach the primary cilium? Although the cloning of the PKD2 gene generated a lot of excitement early on and although we have gained some insight into the biology of polycystin-2, much remains to be learned regarding its physiological and pathophysiological functions.
References
Barr FA, Short B (2003) Golgins in the structure and dynamics of the Golgi apparatus. Curr Opin Cell Biol 15:405–413
Cai Y, Maeda Y, Cedzich A, Torres VE, Wu G, Hayashi T, Mochizuki T, Park JH, Witzgall R, Somlo S (1999) Identification and characterization of polycystin-2, the PKD2 gene product. J Biol Chem 274:28557–28565
Calvet JP (1993) Polycystic kidney disease: primary extracellular matrix abnormality or defective cellular differentiation. Kidney Int 43:101–108
Chen X-Z, Vassilev PM, Basora N, Peng J-B, Nomura H, Segal Y, Brown EM, Reeders ST, Hediger MA, Zhou J (1999) Polycystin-L is a calcium regulated cation channel permeable to calcium ions. Nature 401:383–386
Cole DG (2003) The intraflagellar transport machinery of Chlamydomonas reinhardtii. Traffic 4:435–442
European Polycystic Kidney Disease Consortium (1994) The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell 77:881–894
Gallagher AR, Cedzich A, Gretz N, Somlo S, Witzgall R (2000) The polycystic kidney disease protein PKD2 interacts with Hax-1, a protein associated with the actin cytoskeleton. Proc Natl Acad Sci U S A 97:4017–4022
Gallagher AR, Hidaka S, Gretz N, Witzgall R (2002) Molecular basis of autosomal-dominant polycystic kidney disease. Cell Mol Life Sci 59:682–693
Gillingham AK, Munro S (2003) Long coiled-coil proteins and membrane traffic. Biochim Biophys Acta 1641:71–85
Gilula NB, Satir P (1972) The ciliary necklace. A ciliary membrane specialization. J Cell Biol 53:494–509
González-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF (2001) Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci U S A 98:1182–1187
Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG (2000) Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 408:990–994
Hidaka S, Könecke V, Osten L, Witzgall R (2004) PIGEA-14, a novel coiled-coil protein affecting the intracellular distribution of polycystin-2. J Biol Chem 279:35009–35016
Jensen CG, Poole CA, McGlashan SR, Marko M, Issa ZI, Vujcich KV, Bowser SS (2004) Ultrastructural, tomographic and confocal imaging of the chondrocyte primary cilium in situ. Cell Biol Int 28:101–110
Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S (2002) Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol 4:191–197
Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL (1993) A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci U S A 90:5519–5523
Luo Y, Vassilev PM, Li X, Kawanabe Y, Zhou J (2003) Native polycystin 2 functions as a plasma membrane Ca2+-permeable cation channel in renal epithelia. Mol Cell Biol 23:2600–2607
Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJM, Somlo S (1996) PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272:1339–1342
Montell C (1998) TRP trapped in fly signaling web. Curr Opin Neurobiol 8:389–397
Montell C, Birnbaumer L, Flockerzi V (2002) The TRP channels, a remarkably functional family. Cell 108:595–598
Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AEH, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J (2003) Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33:129–137
Newby LJ, Streets AJ, Zhao Y, Harris PC, Ward CJ, Ong ACM (2002) Identification, characterization, and localization of a novel kidney polycystin-1–polycystin-2 complex. J Biol Chem 277:20763–20773
Pazour GJ (2004) Intraflagellar transport and cilia-dependent renal disease: the ciliary hypothesis of polycystic kidney disease. J Am Soc Nephrol 15:2528–2536
Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB (2002) Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol 12:R378–R380
Praetorius HA, Spring KR (2003) The renal cell primary cilium functions as a flow sensor. Curr Opin Nephrol Hypertens 12:517–520
Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG (1997) PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet 16:179–183
Raychowdhury MK, González-Perrett S, Montalbetti N, Timpanaro GA, Chasan B, Goldmann WH, Stahl S, Cooney A, Goldin E, Cantiello HF (2004) Molecular pathophysiology of mucolipidosis type IV: pH dysregulation of the mucolipin-1 cation channel. Hum Mol Genet 13:617–627
Rogers KK, Wilson PD, Snyder RW, Zhang X, Guo W, Burrow CR, Lipschutz JH (2004) The exocyst localizes to the primary cilium in MDCK cells. Biochem Biophys Res Commun 319:138–143
Scheffers MS, Le H, van der Bent P, Leonhard W, Prins F, Spruit L, Breuning MH, de Heer E, Peters DJM (2002) Distinct subcellular expression of endogenous polycystin-2 in the plasma membrane and Golgi apparatus of MDCK cells. Hum Mol Genet 11:59–67
Tsiokas L, Kim E, Arnould T, Sukhatme VP, Walz G (1997) Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc Natl Acad Sci U S A 94:6965–6970
Vassilev PM, Guo L, Chen X-Z, Segal Y, Peng J-B, Basora N, Babakhanlou H, Cruger G, Kanazirska M, Ye C, Brown EM, Hediger MA, Zhou J (2001) Polycystin-2 is a novel cation channel implicated in defective intracellular Ca2+ homeostasis in polycystic kidney disease. Biochem Biophys Res Commun 282:341–350
Wilson PD (1997) Epithelial cell polarity and disease. Am J Physiol 272:F434–F442
Wilson PD (2004) Polycystic kidney disease. N Engl J Med 350:151–164
Yoder BK, Hou X, Guay-Woodford LM (2002) The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13:2508–2516
Acknowledgements
The data on PIGEA-14 have resulted in large part from the effort of Sumi Hidaka, Vera Könecke and Larissa Osten.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Witzgall, R. Polycystin-2—an intracellular or plasma membrane channel?. Naunyn-Schmiedeberg's Arch Pharmacol 371, 342–347 (2005). https://doi.org/10.1007/s00210-005-1027-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-005-1027-9