- 1Department of Psychiatry, National Center of Neurology and Psychiatry Hospital, Tokyo, Japan
- 2Clinical Research and Education Promotion Division, National Center of Neurology and Psychiatry, Tokyo, Japan
- 3Endowed Institute for Empowering Gifted Minds, Graduate School of Arts and Sciences, The University of Tokyo, Tokyo, Japan
- 4Department of Preventive Intervention for Psychiatric Disorders, National Institute of Mental Health, National Center of Neurology and Psychiatry, Tokyo, Japan
- 5Faculty of Human Development and Culture, Fukushima University, Fukushima, Japan
- 6Department of Pathology of Mental Diseases, National Institute of Mental Health, National Center of Neurology and Psychiatry, Tokyo, Japan
- 7Department of Community Mental Health and Law, National Institute of Mental Health, National Center of Neurology and Psychiatry, Tokyo, Japan
Background: Both impairment and sex differences in social cognition and neurocognition have been documented in schizophrenia. However, whether sex differences exist in the association between social cognition and neurocognition are not known. We aimed to investigate the contribution of areas of neurocognition to theory of mind (ToM) and hostility bias, representing social cognition, according to sex in early course schizophrenia.
Methods: In this cross-sectional study, we assessed neurocognition using the Japanese version of the Brief Assessment of Cognition in Schizophrenia (BACS) and assessed the ToM and hostility bias subdomains of social cognition using the Social Cognition Screening Questionnaire (SCSQ) in 131 participants (65 female, 66 male) diagnosed with schizophrenia within 5 years of onset. Sex differences were analyzed using t-tests. The associations of neurocognitive subdomains with ToM and hostility bias according to sex were analyzed using multiple regression analysis. Results were adjusted by age, estimated premorbid intelligence quotient, and symptomatology.
Results: No sex differences were found in ToM (p = 0.071) or hostility bias (p = 0.057). Higher verbal fluency was significantly associated with higher ToM in females (p < 0.01), whereas higher executive function was significantly associated with higher ToM in males (p < 0.05). Higher verbal fluency was significantly associated with lower hostility bias in females (p < 0.05), whereas neurocognition and hostility bias were not significantly associated in males.
Conclusion: The results suggest that neurocognition associated with social cognition differ according to sex. These differences should be considered for more effective treatment of social cognition.
Introduction
Kraepelin (1919) noted that there is a sex/gender difference in schizophrenia and wrote: “The male sex appears in general to suffer somewhat more frequently from the dementia praecox than the females.” For the past decade, “sex” has been used to refer to biological variables, and “gender” has been used for psychosocial variables (Mendrek and Mancini-Marie, 2016). In recent years, considerable attention has been given to the treatment of social cognition in schizophrenia, including social cognitive training programs and pharmacotherapies, which take into account biological aspects (Javed and Charles, 2018). We use the word “sex” instead of “gender” to indicate that we are discussing the biological aspect in schizophrenia through an assessment of social cognition.
Social cognition is defined as “the mental operations that underlie social interactions, including perceiving, interpreting, and generating responses to the intentions, dispositions, and behaviors of others” (Green et al., 2008). This multidimensional construct is composed of the subdomains of emotional processing, theory of mind (ToM), social perception, and attributional bias (Pinkham et al., 2014). In patients with schizophrenia, impairment of social cognition is considered one of the greatest obstacles to social participation, including that involving interpersonal relationships, education, and employment (Green et al., 2012; Kubota et al., 2021). Over the past decade, considerable attention has been given to the development of new therapies targeting social cognitive deficits as a means to improve functional outcomes in schizophrenia (Green et al., 2019). As some patients in the early course schizophrenia have been reported to respond to both pharmacological and psychosocial treatments (Robinson et al., 1999; Catalan et al., 2018), the optimal time to intervene to prevent future decline might be early in its course (Crumlish et al., 2009; Catalan et al., 2018).
Sex differences have been shown to affect not only functional outcomes (Seeman, 2019), but also social cognition in schizophrenia, and therefore any intervention in early course schizophrenia to improve social cognition should account for these sex differences (Chen et al., 2021). Research on sex differences in social cognition has been conducted to improve functional outcomes. According to previous studies (Buck et al., 2016; Roberts et al., 2016), impairment of social cognition in schizophrenia has a two-factor structure, social cognitive skills including ToM and attributional bias including hostility bias (Buck et al., 2016; Roberts et al., 2016). In terms of sex differences in ToM, there are mixed results indicating either no sex differences or better ToM in females (Abu-Akel and Bo, 2013; Ayesa-Arriola et al., 2014; Navarra-Ventura et al., 2018, 2021; Riecher-Rossler et al., 2018). These conflicting results might be due to differences in disease duration between the studies. A meta-analysis has not yet been carried out, and no conclusion about sex differences in ToM has been reached. To our knowledge, hostility bias has not yet been investigated separately for females and males, so there is a need to investigate potential sex differences.
Treatment of social cognitive functioning should take into account neurocognitive functioning (Deckler et al., 2018; Thibaudeau et al., 2020), and combining the treatment of neurocognitive impairment and social cognitive impairment may improve functional outcomes (Deckler et al., 2018). A number of studies have examined the association between ToM and neurocognition, with a meta-analysis showing that neurocognition such as attention, working memory, episodic memory, speed of processing, language, visuospatial/problem solving and executive functions influence ToM to the same extent, but with no specific neurocognitive area having a pronounced effect on ToM (Thibaudeau et al., 2020). It is possible that several areas influence ToM to the same extent, because that meta-analysis did not account for sex differences. Given that sex differences affect both social cognition and neurocognition (Longenecker et al., 2010; Chen et al., 2021), sex differences need to be taken into account when examining the association between ToM and neurocognition. However, to our knowledge, no studies have done this to date. As for hostility bias, although studies have examined its association with neurocognition (Donohoe et al., 2008; Buck et al., 2017, 2020; Lo and Siu, 2017), the analyses were not conducted separately for female and male subjects. Therefore, the association between hostility bias and neurocognition needs to be examined by sex.
In efforts to facilitate more individualized assessment and treatment of social cognition in patients with early course schizophrenia, this study sought to clarify the association between neurocognitive and social cognitive subdomains in early course schizophrenia by sex. Specifically, first we examined sex differences in ToM and hostility bias in early course schizophrenia and then investigated the contribution of neurocognition to ToM and hostility bias by sex.
Methods and Analysis
Participants
In this cross-sectional study, we recruited 131 patients with schizophrenia (65 female, 66 male) within 5 years of onset who visited the Early Detection and Intervention Center for Schizophrenia (EDICS) at the National Institute of Neurology and Psychiatry between December 2013 and December 2020. Schizophrenia was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5). Patients were excluded if they had concomitant disease with a marked effect on cognition such as dementia, their physician judged that they would potentially be disadvantaged by participating (e.g., relapse, excitement), or the principal investigator deemed their participation inappropriate. Following approval of the study design by the Institutional Review Board of the National Center of Neurology and Psychiatry (Approval No. A2018-139), written informed consent was obtained from all participants. When the participant was a minor (i.e., < 20 years of age), written consent was obtained from a parent or guardian, with additional written assent obtained from patients aged 16–19 years.
Measures
The Social Cognition Screening Questionnaire (SCSQ) was used to assess social cognition. This quick and objectively scored instrument was designed to help busy clinicians practicing in real-world clinical settings to conduct a standard assessment of treatment (Roberts et al., 2016). It consists of five domains: verbal memory, schematic inference, ToM, metacognition, and hostility bias. For each domain except for hostility bias, higher scores indicate higher performance. We used the scores for ToM and hostility bias to represent social cognition in this study. The reliability and validity of the Japanese version of the SCSQ have been verified. The validity of ToM and hostility bias domains were examined by investigating their correlation with the Hinting task, the Ambiguous Intention Hostility Questionnaire (AIHQ), and the Social Functioning Scale, respectively (Roberts et al., 2011; Kanie et al., 2014).
The Japanese version of the Brief Assessment of Cognition in Schizophrenia (BACS) was used to assess neurocognition. The BACS was developed to assess cognitive impairment in schizophrenia and is currently widely used to assess psychiatric disorders. It consists of 6 domains: verbal memory, working memory, motor speed, attention, verbal fluency, and executive functions. Higher scores indicate higher performance. The reliability and validity of the Japanese version have been verified (Keefe et al., 2004; Kaneda et al., 2007).
The Positive and Negative Syndrome Scale (PANSS) was used to assess overall psychiatric symptoms in schizophrenia during an interview. This scale includes a positive symptom subscale, negative symptom subscale, and general psychopathology subscale. Each item is rated from 1 (absent) to 7 (extreme). The Japanese version has been validated (Kay et al., 1987; Igarashi et al., 1998; Hashimoto et al., 2020).
The 25-item short version of the Japanese Adult Reading Test (JART25) was used to assess estimated pre-morbid IQ. The JART is the Japanese version of the National Adult Reading Test (Nelson and O’Connell, 1978). The JART25 tests the reading of 25 kanji characters (Matsuoka et al., 2006).
The Global Assessment of Functioning (GAF) was administered to provide a global assessment of the patient’s condition, ranging from good mental health to severe psychopathology. It is a general rather than diagnostic-specific scoring system. The 100-point GAF scale is divided into 10-point intervals (sections) (American Psychiatric Association [APA], 1994).
Lastly, factors affecting neurocognition and social cognition were examined, including age, disease duration, years of education, estimated pre-morbid IQ, and psychiatric symptoms (Pinkham et al., 2017; Chen et al., 2021; Navarra-Ventura et al., 2021).
Statistical Analysis
Variables are described using means and standard deviations (SD). Results for the social cognitive subdomains, neurocognitive subdomains, psychiatric symptoms, and estimated pre-morbid IQ were compared between the female and male participants. Continuous variables were compared using standardized differences and t-tests.
Multivariable regression models were used to examine the association between each neurocognitive subdomain and each social cognitive subdomain in female and male participants. Multivariate analyses were performed with psychiatric symptoms, age, estimated pre-morbid IQ, and neurocognition as independent variables. Disease duration and years of education were excluded from the multiple regression analyses because the participants were within 5 years of onset and because estimated pre-morbid IQ and years of education overlapped. Results were considered statistically significant at p < 0.05. All statistical analyses were performed using IBM SPSS Statistics version 26.
Results
Sociodemographic Variables
Means and standard deviations for demographic variables, clinical variables, and PANSS scores are shown in Table 1. There were no sex differences in factors known to affect neurocognition and social cognition such as age, disease duration, years of education, estimated pre-morbid IQ and psychiatric symptoms.
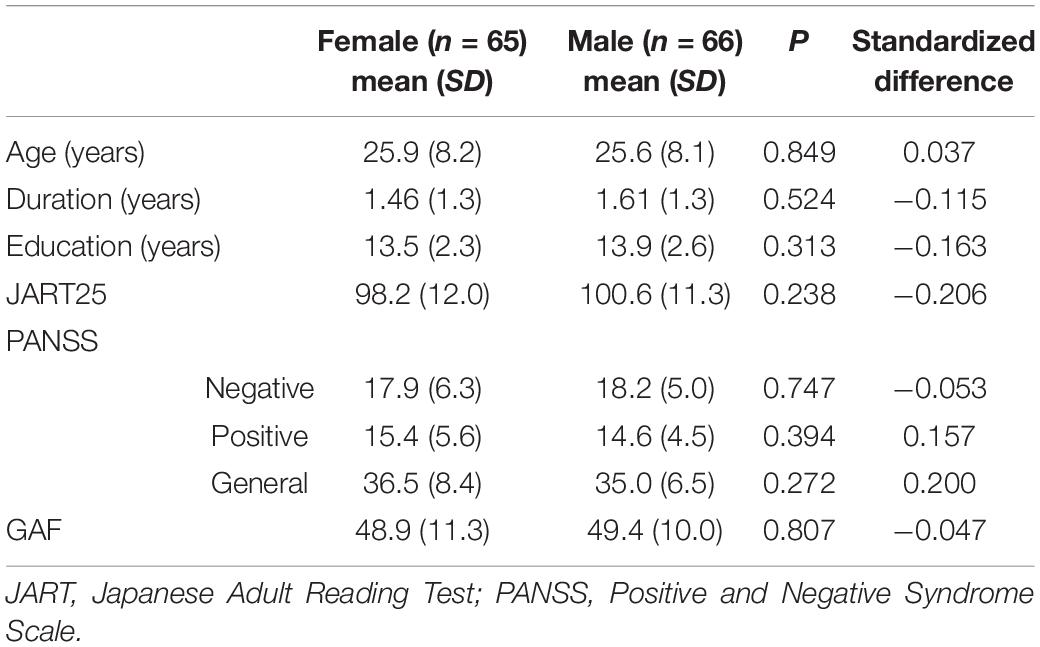
Table 1. Sociodemographic variables and clinical data for participants with early course schizophrenia.
Neurocognitive and Social Cognitive Tasks
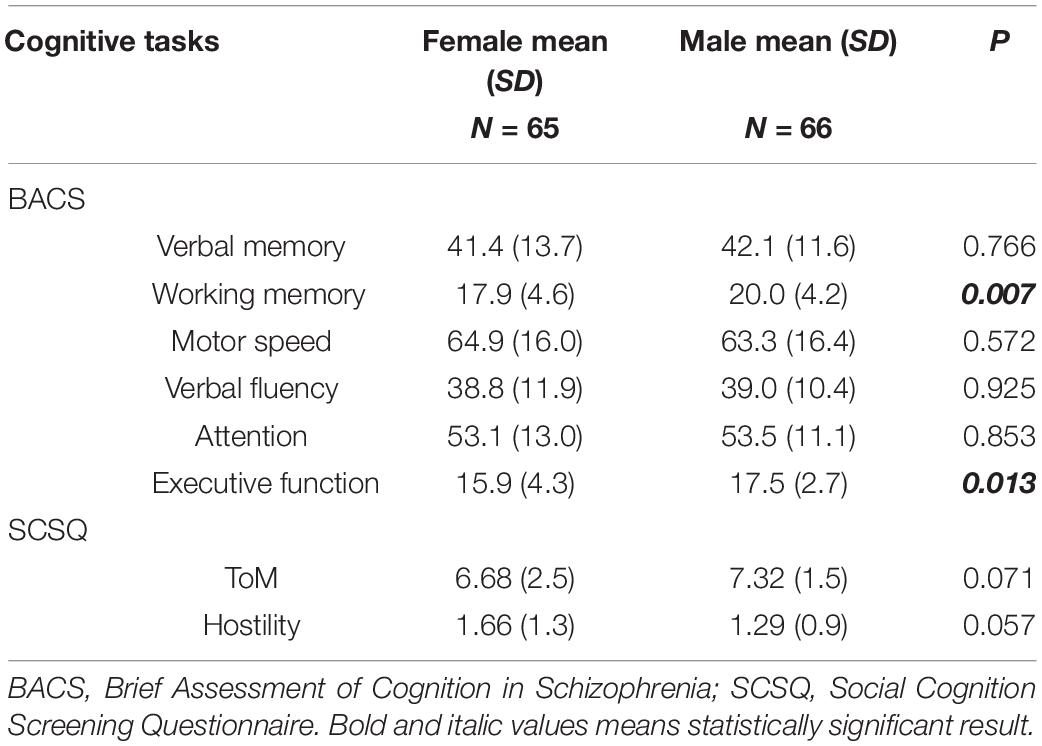
Results of the BACS and SCSQ are shown in Table 2. Males had significantly higher scores than females for executive function and working memory, but there was no significant sex difference for ToM or hostility bias.
Association Between Neurocognition and Social Cognition
As shown in Table 3, the association between neurocognition and social cognition was assessed after adjusting for age, JART25, and PANSS scores. The results showed statistically significant associations between some of the indicators and ToM and hostility bias, independent of other variables. Higher verbal fluency was significantly associated with higher ToM in females (p < 0.01) but not in males (p = 0.74), whereas higher executive function was significantly associated with higher ToM in males (p < 0.05) but not in females (p = 0.92). Higher verbal fluency was significantly associated with lower hostility bias in females (p < 0.05) but not in males (p = 0.68). None of the neurocognitive subdomains were significantly associated with hostility bias in males. For psychiatric symptoms as measured by the PANSS, none of its three domains (Positive scale, Negative scale, and General Psychopathology scale) was associated with ToM or hostility bias. In all regression models, multicollinearity between predictors was checked. Variance inflation factors ranged from 1.141 to 3.460, all of which fell below the threshold of 10 for adverse multicollinearity.
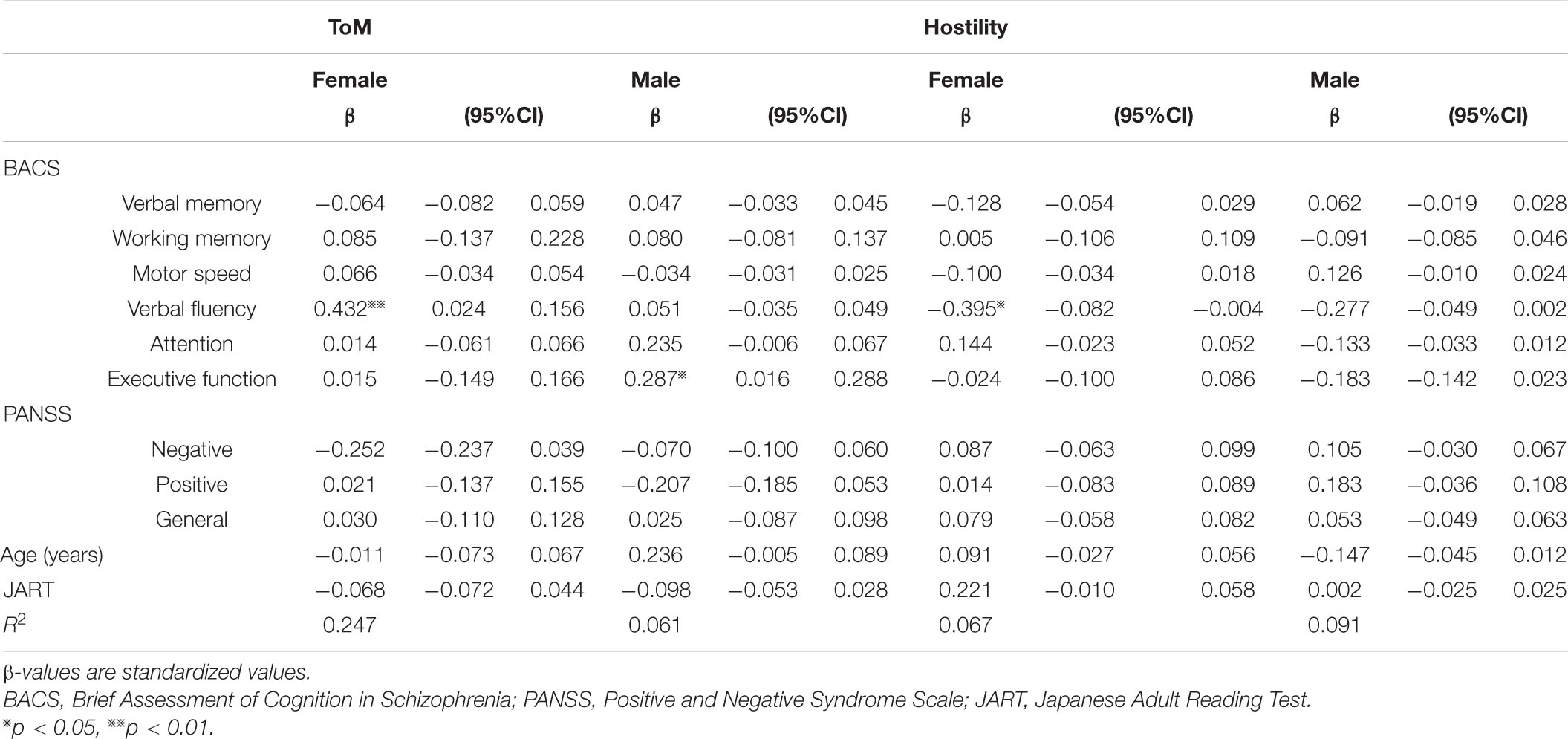
Table 3. Association between neurocognitive performance and symptoms with ToM and hostility bias (multivariate analysis).
Discussion
Our examination of sex differences in the social cognitive subdomains of ToM and hostility bias revealed no such differences in patients with early course schizophrenia. Previous studies on sex differences in ToM in schizophrenia reported either no sex differences in ToM (Ayesa-Arriola et al., 2014; Navarra-Ventura et al., 2018, 2021; Riecher-Rossler et al., 2018) or better ToM in females (Abu-Akel and Bo, 2013). This inconsistency in findings might be related to differences in disease duration between the studies. To address this, in the present study, we examined patients with schizophrenia within 5 years of onset. Our study also had no significant sex differences in sociodemographic variables and thus might provide more accurate results for examining sex differences. Although no previous studies have compared sex differences in hostility bias, one study examining whether sex affects hostility bias (Pinkham et al., 2017) had a finding consistent with ours, namely, that sex does not affect hostility bias. We found that there were no sex differences in ToM and hostility bias. In contrast, healthy individuals in previous studies have shown sex differences. These differences can be explained by the effect of estrogen. In the healthy individuals, females have higher ToM (Baron-Cohen et al., 2005; Navarra-Ventura et al., 2021) and lower hostility bias (Baron-Cohen, 2004; Combs et al., 2007; Runions and Keating, 2007). Lower estrogen is associated with lower ToM (Oldershaw et al., 2010; Abu-Akel and Bo, 2013) and higher Hostility (Riecher-Rossler and Kulkarni, 2011). Gonadal hormone levels including estrogen is known to be lower in schizophrenia in 92% of females and 27.7% of males (Howes et al., 2007). Since the decrease in estrogen is greater in females, it is possible that the sex difference observed in the healthy individuals are not present in schizophrenia. Therefore, it was suggested that treatment with estrogen may be effective against ToM and hostility bias.
Interestingly, we found that verbal fluency contributed significantly to ToM in females (p < 0.01), whereas executive function contributed significantly to ToM in males (p < 0.05). This is the first study to examine the association between ToM and neurocognition in schizophrenia according to sex. To our knowledge, only one study has examined the association between ToM and brain function in schizophrenia by sex (Walsh-Messinger et al., 2019). ToM in females with schizophrenia was found to be associated with the limbic system, whereas ToM in males with schizophrenia was associated with the frontal lobe. In people with schizophrenia and healthy controls, previous studies have shown an association between verbal fluency and the limbic system and between executive function and the frontal lobe (Pihlajamaki et al., 2000; Zhu et al., 2010; Morimoto et al., 2018). Together, the results of our study and previous studies indicate that limbic-based verbal fluency is an important predictor of ToM in females and frontal-based executive function in males. These implications are useful in planning treatment for cognitive rehabilitation. Cognitive rehabilitation aimed at improving ToM should account for deficits in executive function in males and verbal fluency in females. In some cases, it might be helpful to provide treatment for executive function and verbal fluency skills before treatment for ToM.
For hostility bias, higher verbal fluency was significantly associated with lower hostility bias in females (p < 0.05), whereas none of the neurocognitive subdomains was significantly associated with hostility bias in males, although verbal fluency may have a marked contribution (p = 0.068). No sex differences were found in the association between neurocognition and hostility bias. As far as we know, no previous studies have examined the association between neurocognition and hostility bias in schizophrenia in terms of sex differences, although some studies have consistently reported that the neurocognitive subdomains that contribute to attributional bias are verbal-related ones, such as verbal fluency (Lo and Siu, 2017) and verbal IQ (Donohoe et al., 2008; Buck et al., 2017). Our results also showed that verbal-related neurocognitive subdomains tended to be associated with hostility bias. Previous studies examining the neural basis of hostility in schizophrenia have pointed out an association between hostility and the limbic system (Hoptman et al., 2010; Del Bene et al., 2016; Perlini et al., 2018). As mentioned above, an association also exists between verbal fluency and the limbic system. In fact, hostility and verbal fluency in schizophrenia have a common neural basis, which is in line with our results. Before conducting this study, we expected to find sex differences in the association between neurocognitive subdomains and hostility bias, but we found no such differences. Therefore, female and male patients alike might benefit from similar cognitive rehabilitation strategies to treat hostility bias. In addition to the lack of sex differences in ToM and hostility bias observed in schizophrenia as mentioned above, the associations between neurocognition and ToM and neurocognition and hostility bias also suggest that treatment with estrogen may benefit ToM and hostility bias in both females and males. Previous studies have found that estrogen modifies frontal lobe and limbic system activity during emotion perception tasks, a subdomain of social cognition (Ji et al., 2016; Javed and Charles, 2018). Estrogen is also associated with frontal-based executive function and limbic-based verbal fluency (Ko et al., 2006). Estrogen not only improves frontal-based executive function and limbic-based verbal fluency, but may also be effective in treating ToM and hostility bias, as well as emotion perception. Combining estrogen with neurocognitive and social cognitive rehabilitation may facilitate the treatment of ToM and hostility bias. Further studies focusing on sex differences in hormones might be worthwhile to improve the rehabilitation on social cognition (Sumiyoshi et al., 1997; Gonzalez-Rodriguez et al., 2020). Furthermore, female patients are said to have better prognosis. Our hope is that studying sex differences will uncover critical elements of good outcome thus leading to interventions that will benefit both females and males (Seeman, 2019). By considering associations with cognitive function, it may be possible to provide interventions that take account of sex differences in prognosis.
The limitations of this study are as follows. First, as this was not a longitudinal study, it cannot establish causality. Second, antipsychotic medications that our patients were taking might affect cognitive function; however, the recent literature generally does not report any significant effects of antipsychotics on social cognition (Hempel et al., 2010; Catalan et al., 2018). Third, we assessed ToM and hostility bias using the SCSQ, but it would have been beneficial to have examined other subdomains of social cognition as well. Fourth, in this study, the social cognitive assessment relies on SCSQ which is used as a screening tool. However, ToM and hostility bias domains scores of SCSQ were well-correlated with those of standard measures such as the Hinting task and AIHQ (r = 0.52, P = 0.0001 for ToM and r = 0.34, P = 0.05 for hostility bias) (Kanie et al., 2014). We therefore do not believe this undermines the validity of our results. To date, there is no consensus on what kind of scale is appropriate for measuring social cognitive function among the Japanese, and we are currently conducting research to clarify this (Kubota et al., 2021; Okano et al., 2021). We believe it is desirable to use tests that have reached consensus on this. Lastly, this study did not have a healthy control group to compare our results with. Therefore, it is difficult to state whether these results are specific to schizophrenia or reflect the sex differences found in the general population. In order to link our findings to rehabilitation, the differences need to be fully clarified.
Conclusion
In conclusion, we found sex differences in the associations between neurocognition and ToM and neurocognition and hostility bias in early course schizophrenia, despite the absence of sex differences in ToM and hostility bias. These results suggest that sex differences in neurocognition related to social cognition need to be taken into account to treat social cognition more effectively. These results can be adapted to patients with early course schizophrenia and contribute to the promotion of future treatments targeting social cognition.
Data Availability Statement
The datasets presented in this article are not readily available because participants of this study did not agree for their data to be shared publicly. Requests to access the datasets should be directed to RK, ryo-okubo@ncnp.go.jp.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of the National Center of Neurology and Psychiatry. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
RK, RO, SI, and NY contributed to writing—original draft preparation. RO, SI, and NY contributed to supervision. All authors contributed to conceptualization and writing—review and editing, read and agreed to the published version of the manuscript.
Funding
This study was supported by AMED under Grant Number JP20dk0307092 and an Intramural Research Grant (25-S7) for Neurological and Psychiatric Disorders of NCNP.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abu-Akel, A., and Bo, S. (2013). Superior mentalizing abilities of female patients with schizophrenia. Psychiatry Res. 210, 794–799. doi: 10.1016/j.psychres.2013.09.013
American Psychiatric Association [APA] (1994). Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), 4th Edn. Washington DC: American Psychiatric Association.
Ayesa-Arriola, R., Rodriguez-Sanchez, J. M., Gomez-Ruiz, E., Roiz-Santianez, R., Reeves, L. L., and Crespo-Facorro, B. (2014). No sex differences in neuropsychological performance in first episode psychosis patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 48, 149–154. doi: 10.1016/j.pnpbp.2013.09.009
Baron-Cohen, S., Knickmeyer, R. C., and Belmonte, M. K. (2005). Sex differences in the brain: implications for explaining autism. Science 310, 819–823. doi: 10.1126/science.1115455
Buck, B., Browne, J., Gagen, E. C., and Penn, D. L. (2020). Hostile attribution bias in schizophrenia-spectrum disorders: narrative review of the literature and persisting questions. J. Ment. Health 1–18. [Epub ahead of print]. doi: 10.1080/09638237.2020.1739240
Buck, B., Iwanski, C., Healey, K. M., Green, M. F., Horan, W. P., Kern, R. S., et al. (2017). Improving measurement of attributional style in schizophrenia; a psychometric evaluation of the Ambiguous Intentions Hostility Questionnaire (AIHQ). J. Psychiatr. Res. 89, 48–54. doi: 10.1016/j.jpsychires.2017.01.004
Buck, B. E., Healey, K. M., Gagen, E. C., Roberts, D. L., and Penn, D. L. (2016). Social cognition in schizophrenia: factor structure, clinical and functional correlates. J. Ment. Health 25, 330–337. doi: 10.3109/09638237.2015.1124397
Catalan, A., Angosto, V., Diaz, A., Martinez, N., Guede, D., Pereda, M., et al. (2018). The relationship between theory of mind deficits and neurocognition in first episode-psychosis. Psychiatry Res. 268, 361–367. doi: 10.1016/j.psychres.2018.06.066
Chen, S., Liu, Y., Liu, D., Zhang, G., and Wu, X. (2021). The difference of social cognitive and neurocognitive performance between patients with schizophrenia at different stages and influencing factors. Schizophr. Res. Cogn. 24:100195. doi: 10.1016/j.scog.2021.100195
Combs, D. R., Penn, D. L., Wicher, M., and Waldheter, E. (2007). The Ambiguous Intentions Hostility Questionnaire (AIHQ): a new measure for evaluating hostile social-cognitive biases in paranoia. Cogn. Neuropsychiatry 12, 128–143. doi: 10.1080/13546800600787854
Crumlish, N., Whitty, P., Clarke, M., Browne, S., Kamali, M., Gervin, M., et al. (2009). Beyond the critical period: longitudinal study of 8-year outcome in first-episode non-affective psychosis. Br. J. Psychiatry 194, 18–24. doi: 10.1192/bjp.bp.107.048942
Deckler, E., Hodgins, G. E., Pinkham, A. E., Penn, D. L., and Harvey, P. D. (2018). Social cognition and neurocognition in schizophrenia and healthy controls: intercorrelations of performance and effects of manipulations aimed at increasing task difficulty. Front. Psychiatry 9:356. doi: 10.3389/fpsyt.2018.00356
Del Bene, V. A., Foxe, J. J., Ross, L. A., Krakowski, M. I., Czobor, P., and De Sanctis, P. (2016). Neuroanatomical abnormalities in violent individuals with and without a diagnosis of schizophrenia. PLoS One 11:e0168100. doi: 10.1371/journal.pone.0168100
Donohoe, G., Spoletini, I., Mcglade, N., Behan, C., Hayden, J., O’donoghue, T., et al. (2008). Are relational style and neuropsychological performance predictors of social attributions in chronic schizophrenia? Psychiatry Res. 161, 19–27. doi: 10.1016/j.psychres.2007.10.001
Gonzalez-Rodriguez, A., Guardia, A., Alvarez Pedrero, A., Betriu, M., Cobo, J., Acebillo, S., et al. (2020). Women with schizophrenia over the life span: health promotion, treatment and outcomes. Int. J. Environ. Res. Public Health 17:5594. doi: 10.3390/ijerph17155594
Green, M. F., Hellemann, G., Horan, W. P., Lee, J., and Wynn, J. K. (2012). From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Arch. Gen. Psychiatry 69, 1216–1224. doi: 10.1001/archgenpsychiatry.2012.652
Green, M. F., Horan, W. P., and Lee, J. (2019). Nonsocial and social cognition in schizophrenia: current evidence and future directions. World Psychiatry 18, 146–161. doi: 10.1002/wps.20624
Green, M. F., Penn, D. L., Bentall, R., Carpenter, W. T., Gaebel, W., Gur, R. C., et al. (2008). Social cognition in schizophrenia: an NIMH workshop on definitions, assessment, and research opportunities. Schizophr. Bull. 34, 1211–1220. doi: 10.1093/schbul/sbm145
Hashimoto, N., Takahashi, K., Fujisawa, D., Aoyama, K., Nakagawa, A., Okamura, N., et al. (2020). A pilot validation study of the Japanese translation of the positive and negative syndrome scale (PANSS). Asian J. Psychiatr. 54:102210. doi: 10.1016/j.ajp.2020.102210
Hempel, R. J., Dekker, J. A., Van Beveren, N. J., Tulen, J. H., and Hengeveld, M. W. (2010). The effect of antipsychotic medication on facial affect recognition in schizophrenia: a review. Psychiatry Res. 178, 1–9. doi: 10.1016/j.psychres.2008.07.025
Hoptman, M. J., D’angelo, D., Catalano, D., Mauro, C. J., Shehzad, Z. E., Kelly, A. M., et al. (2010). Amygdalofrontal functional disconnectivity and aggression in schizophrenia. Schizophr. Bull. 36, 1020–1028. doi: 10.1093/schbul/sbp012
Howes, O. D., Wheeler, M. J., Pilowsky, L. S., Landau, S., Murray, R. M., and Smith, S. (2007). Sexual function and gonadal hormones in patients taking antipsychotic treatment for schizophrenia or schizoaffective disorder. J. Clin. Psychiatry 68, 361–367. doi: 10.4088/jcp.v68n0302
Igarashi, Y., Hayashi, N., Yamashina, M., Otsuka, N., Kuroki, N., Anzai, N., et al. (1998). Interrater reliability of the Japanese version of the positive and negative syndrome scale and the appraisal of its training effect. Psychiatry Clin. Neurosci. 52, 467–470. doi: 10.1046/j.1440-1819.1998.00425.x
Javed, A., and Charles, A. (2018). The importance of social cognition in improving functional outcomes in schizophrenia. Front. Psychiatry 9:157. doi: 10.3389/fpsyt.2018.00157
Ji, E., Weickert, C. S., Lenroot, R., Kindler, J., Skilleter, A. J., Vercammen, A., et al. (2016). Adjunctive selective estrogen receptor modulator increases neural activity in the hippocampus and inferior frontal gyrus during emotional face recognition in schizophrenia. Transl. Psychiatry 6:e795. doi: 10.1038/tp.2016.59
Kaneda, Y., Sumiyoshi, T., Keefe, R., Ishimoto, Y., Numata, S., and Ohmori, T. (2007). Brief assessment of cognition in schizophrenia: validation of the Japanese version. Psychiatry Clin. Neurosci. 61, 602–609. doi: 10.1111/j.1440-1819.2007.01725.x
Kanie, A., Hagiya, K., Ashida, S., Pu, S., Kaneko, K., Mogami, T., et al. (2014). New instrument for measuring multiple domains of social cognition: construct validity of the Social Cognition Screening Questionnaire (Japanese version). Psychiatry Clin. Neurosci. 68, 701–711. doi: 10.1111/pcn.12181
Kay, S. R., Fiszbein, A., and Opler, L. A. (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 13, 261–276. doi: 10.1093/schbul/13.2.261
Keefe, R. S., Goldberg, T. E., Harvey, P. D., Gold, J. M., Poe, M. P., and Coughenour, L. (2004). The brief assessment of cognition in schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr. Res. 68, 283–297. doi: 10.1016/j.schres.2003.09.011
Ko, Y. H., Joe, S. H., Cho, W., Park, J. H., Lee, J. J., Jung, I. K., et al. (2006). Estrogen, cognitive function and negative symptoms in female schizophrenia. Neuropsychobiology 53, 169–175. doi: 10.1159/000093780
Kubota, R., Okubo, R., Akiyama, H., Okano, H., Ikezawa, S., Miyazaki, A., et al. (2021). Study protocol: the evaluation study for social cognition measures in Japan (ESCoM). J. Pers. Med. 11:667. doi: 10.3390/jpm11070667
Lo, P. M. T., and Siu, A. M. H. (2017). Assessing social cognition of persons with schizophrenia in a Chinese population: a pilot study. Front. Psychiatry 8:302. doi: 10.3389/fpsyt.2017.00302
Longenecker, J., Dickinson, D., Weinberger, D. R., and Elvevag, B. (2010). Cognitive differences between men and women: a comparison of patients with schizophrenia and healthy volunteers. Schizophr. Res. 120, 234–235. doi: 10.1016/j.schres.2009.12.009
Matsuoka, K., Uno, M., Kasai, K., Koyama, K., and Kim, Y. (2006). Estimation of premorbid IQ in individuals with Alzheimer’s disease using Japanese ideographic script (Kanji) compound words: Japanese version of National Adult Reading Test. Psychiatry Clin. Neurosci. 60, 332–339. doi: 10.1111/j.1440-1819.2006.01510.x
Mendrek, A., and Mancini-Marie, A. (2016). Sex/gender differences in the brain and cognition in schizophrenia. Neurosci. Biobehav. Rev. 67, 57–78. doi: 10.1016/j.neubiorev.2015.10.013
Morimoto, T., Matsuda, Y., Matsuoka, K., Yasuno, F., Ikebuchi, E., Kameda, H., et al. (2018). Computer-assisted cognitive remediation therapy increases hippocampal volume in patients with schizophrenia: a randomized controlled trial. BMC Psychiatry 18:83. doi: 10.1186/s12888-018-1667-1
Navarra-Ventura, G., Fernandez-Gonzalo, S., Turon, M., Pousa, E., Palao, D., Cardoner, N., et al. (2018). Gender differences in social cognition: a cross-sectional pilot study of recently diagnosed patients with schizophrenia and healthy subjects. Can. J. Psychiatry 63, 538–546. doi: 10.1177/0706743717746661
Navarra-Ventura, G., Vicent-Gil, M., Serra-Blasco, M., Massons, C., Crosas, J. M., Cobo, J., et al. (2021). Group and sex differences in social cognition in bipolar disorder, schizophrenia/schizoaffective disorder and healthy people. Compr. Psychiatry 109:152258. doi: 10.1016/j.comppsych.2021.152258
Nelson, H. E., and O’Connell, A. (1978). Dementia: the estimation of premorbid intelligence levels using the New Adult Reading Test. Cortex 14, 234–244. doi: 10.1016/s0010-9452(78)80049-5
Okano, H., Kubota, R., Okubo, R., Hashimoto, N., Ikezawa, S., Toyomaki, A., et al. (2021). Evaluation of social cognition measures for Japanese patients with schizophrenia using an expert panel and modified Delphi method. J. Pers. Med. 11:275. doi: 10.3390/jpm11040275
Oldershaw, A., Hambrook, D., Tchanturia, K., Treasure, J., and Schmidt, U. (2010). Emotional theory of mind and emotional awareness in recovered anorexia nervosa patients. Psychosom. Med. 72, 73–79. doi: 10.1097/PSY.0b013e3181c6c7ca
Perlini, C., Bellani, M., Besteher, B., Nenadic, I., and Brambilla, P. (2018). The neural basis of hostility-related dimensions in schizophrenia. Epidemiol. Psychiatr. Sci. 27, 546–551. doi: 10.1017/S2045796018000525
Pihlajamaki, M., Tanila, H., Hanninen, T., Kononen, M., Laakso, M., Partanen, K., et al. (2000). Verbal fluency activates the left medial temporal lobe: a functional magnetic resonance imaging study. Ann. Neurol. 47, 470–476. doi: 10.1002/1531-8249(200004)47:4<470::aid-ana10>3.0.co;2-m
Pinkham, A. E., Kelsven, S., Kouros, C., Harvey, P. D., and Penn, D. L. (2017). The effect of age, race, and sex on social cognitive performance in individuals with schizophrenia. J. Nerv. Ment. Dis. 205, 346–352. doi: 10.1097/nmd.0000000000000654
Pinkham, A. E., Penn, D. L., Green, M. F., Buck, B., Healey, K., and Harvey, P. D. (2014). The social cognition psychometric evaluation study: results of the expert survey and RAND panel. Schizophr. Bull. 40, 813–823. doi: 10.1093/schbul/sbt081
Riecher-Rossler, A., Butler, S., and Kulkarni, J. (2018). Sex and gender differences in schizophrenic psychoses-a critical review. Arch. Womens Ment. Health 21, 627–648. doi: 10.1007/s00737-018-0847-9
Riecher-Rossler, A., and Kulkarni, J. (2011). Estrogens and gonadal function in schizophrenia and related psychoses. Curr. Top. Behav. Neurosci. 8, 155–171. doi: 10.1007/7854_2010_100
Roberts, D. L., Fiszdon, J., and Tek, C. (2011). Ecological validity of the social cognition screening questionnaire (SCSQ). Schizophr. Bull. 37(Suppl. 1), 280–280.
Roberts, D. L., Penn, D. L., Combs, D. R., and Ebook Central Academic (2016). Social Cognition and Interaction Training (SCIT): Group Psychotherapy for Schizophrenia and Other Psychotic Disorders Clinician Guide. Oxford: Oxford University Press.
Robinson, D. G., Woerner, M. G., Alvir, J. M., Geisler, S., Koreen, A., Sheitman, B., et al. (1999). Predictors of treatment response from a first episode of schizophrenia or schizoaffective disorder. Am. J. Psychiatry 156, 544–549.
Runions, K. C., and Keating, D. P. (2007). Young children’s social information processing: family antecedents and behavioral correlates. Dev. Psychol. 43, 838–849. doi: 10.1037/0012-1649.43.4.838
Seeman, M. V. (2019). Does gender influence outcome in schizophrenia? Psychiatr. Q. 90, 173–184. doi: 10.1007/s11126-018-9619-y
Sumiyoshi, T., Hasegawa, M., Jayathilake, K., and Meltzer, H. Y. (1997). Sex differences in plasma homovanillic acid levels in schizophrenia and normal controls: relation to neuroleptic resistance. Biol. Psychiatry 41, 560–566. doi: 10.1016/s0006-3223(96)00099-6
Thibaudeau, E., Achim, A. M., Parent, C., Turcotte, M., and Cellard, C. (2020). A meta-analysis of the associations between theory of mind and neurocognition in schizophrenia. Schizophr. Res. 216, 118–128. doi: 10.1016/j.schres.2019.12.017
Walsh-Messinger, J., Stepanek, C., Wiedemann, J., Goetz, D., Goetz, R. R., and Malaspina, D. (2019). Normal sexual dimorphism in theory of mind circuitry is reversed in schizophrenia. Soc. Neurosci. 14, 583–593. doi: 10.1080/17470919.2018.1536613
Keywords: schizophrenia, social cognition, neurocognition, theory of mind (ToM), hostility bias, sex difference
Citation: Kubota R, Okubo R, Ikezawa S, Matsui M, Adachi L, Wada A, Fujimaki C, Yamada Y, Saeki K, Sumiyoshi C, Kikuchi A, Omachi Y, Takeda K, Hashimoto R, Sumiyoshi T and Yoshimura N (2022) Sex Differences in Social Cognition and Association of Social Cognition and Neurocognition in Early Course Schizophrenia. Front. Psychol. 13:867468. doi: 10.3389/fpsyg.2022.867468
Received: 01 February 2022; Accepted: 25 March 2022;
Published: 15 April 2022.
Edited by:
Jeffrey S. Bedwell, University of Central Florida, United StatesReviewed by:
David Eugene Vance, University of Alabama at Birmingham, United StatesDelphine Raucher-Chene, Douglas Mental Health University Institute, Canada
Copyright © 2022 Kubota, Okubo, Ikezawa, Matsui, Adachi, Wada, Fujimaki, Yamada, Saeki, Sumiyoshi, Kikuchi, Omachi, Takeda, Hashimoto, Sumiyoshi and Yoshimura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ryo Okubo, ryo-okubo@ncnp.go.jp; Satoru Ikezawa, satoru-ikezawa@g.ecc.u-tokyo.ac.jp
†These authors have contributed equally to this work
 Ryotaro Kubota
Ryotaro Kubota Ryo Okubo
Ryo Okubo Satoru Ikezawa
Satoru Ikezawa Makoto Matsui1
Makoto Matsui1 Yuji Yamada
Yuji Yamada Koji Saeki
Koji Saeki Chika Sumiyoshi
Chika Sumiyoshi Akiko Kikuchi
Akiko Kikuchi Yoshie Omachi
Yoshie Omachi Kazuyoshi Takeda
Kazuyoshi Takeda Ryota Hashimoto
Ryota Hashimoto Tomiki Sumiyoshi
Tomiki Sumiyoshi