Examining the immune signatures of SARS-CoV-2 infection in pregnancy and the impact on neurodevelopment: Protocol of the SIGNATURE longitudinal study
- 1Mental Health Unit, Virgen del Rocio University Hospital, Seville, Spain
- 2Translational Psychiatry Group, Seville Biomedical Research Institute (IBiS), Seville, Spain
- 3Spanish Network for Research in Mental Health CIBERSAM, ISCIII, Madrid, Spain
- 4Department of Psychiatry, University of Seville, Seville, Spain
- 5Department of Genetics, Reproduction and Maternal-Fetal Medicine, University Hospital Virgen del Rocío, Seville, Spain
- 6Clinical Unit of Infectious Diseases, Microbiology and Preventive Medicine, Seville Biomedical Research Institute (IBiS), Virgen del Rocío University Hospital, CSIC, University of Seville, Seville, Spain
- 7Congenital Immunity Disorders Group de Alteraciones Congénitas de Inmunidad, Seville Biomedical Research Institute, Seville, Spain
- 8Pediatrics, Infectious Diseases and Immunology Department, University Hospital Virgen del Rocío, Sevilla, Spain
- 9Service of General Psychiatry, Lausanne University Hospital (CHUV), Lausanne, Switzerland
- 10Department of Psychosis Studies, Institute of Psychiatry, Psychology & Neuroscience, King's College London, London, United Kingdom
- 11Department of Pediatrics, Virgen del Rocío University Hospital / Institute of Biomedicine of Seville (IBiS), Seville, Spain
- 12Department of Medical Physiology and Biophysics, Seville Biomedical Research Institute (IBiS), Virgen del Rocío University Hospital, CSIC, University of Seville, Seville, Spain
- 13Department of family medicine, Virgen del Rocío University Hospital, Primary Care Health Centers, Seville, Spain
- 14Department of Psychiatry, University Hospital Marqués de Valdecilla - Instituto de Investigación Marqués de Valdecilla (IDIVAL), Santander, Spain
- 15Department of Evolutionary Biology, Ecology and Environmental Sciences, Faculty of Biology, University of Barcelona (UB), Barcelona, Spain
- 16Department of Pharmacology & Toxicology, Faculty of Medicine, Universidad Complutense Madrid, CIBERSAM, Imas12, IUINQ, Madrid, Spain
- 17Viral Diseases and Infections in Immunodeficiencies Research Group, Institute of Biomedicine of Seville (IBiS), Virgen del Rocío University Hospital/CSIC/University of Seville, Seville, Spain
The COVID-19 pandemic represents a valuable opportunity to carry out cohort studies that allow us to advance our knowledge on pathophysiological mechanisms of neuropsychiatric diseases. One of these opportunities is the study of the relationships between inflammation, brain development and an increased risk of suffering neuropsychiatric disorders. Based on the hypothesis that neuroinflammation during early stages of life is associated with neurodevelopmental disorders and confers a greater risk of developing neuropsychiatric disorders, we propose a cohort study of SARS-CoV-2-infected pregnant women and their newborns. The main objective of SIGNATURE project is to explore how the presence of prenatal SARS-CoV-2 infection and other non-infectious stressors generates an abnormal inflammatory activity in the newborn. The cohort of women during the COVID-19 pandemic will be psychological and biological monitored during their pregnancy, delivery, childbirth and postpartum. The biological information of the umbilical cord (foetus blood) and peripheral blood from the mother will be obtained after childbirth. These samples and the clinical characterisation of the cohort of mothers and newborns, are tremendously valuable at this time. This is a protocol report and no analyses have been conducted yet, being currently at, our study is in the recruitment process step. At the time of this publication, we have identified 1,060 SARS-CoV-2 infected mothers and all have already given birth. From the total of identified mothers, we have recruited 537 SARS-COV-2 infected women and all of them have completed the mental health assessment during pregnancy. We have collected biological samples from 119 mothers and babies. Additionally, we have recruited 390 non-infected pregnant women.
Introduction
Foetus exposure to inflammation and its characteristics and severity are determining risk factors for neurodevelopmental disorders and eventually neuropsychiatric diseases [e.g., schizophrenia, autism spectrum disorders (ASD), attention deficit hyperactivity disorder] (rev. in Hagberg et al., 2015). A recent meta-analysis suggests that maternal infection during pregnancy confers an increased risk for ASD in offspring (1). Existing literature on the link between epidemics, pandemics and the increased incidence of schizophrenia spectrum disorders suggests that exposure to SARS-CoV-2 in the utero may put children born during this pandemic at risk for specific neuropsychiatric outcomes (2).
There are currently 100 million pregnant women in the world who are susceptible to being infected by SARS-CoV-2. Cohort studies of pregnant women and their descendants offer an unique opportunity to explore and anticipate the effects of SARS-CoV-2 infection on relevant neurodevelopmental aspects (3). Epidemiological studies, also carried out in pandemic periods, support the idea that the activation of the immune system during pregnancy has important consequences for foetal neurodevelopment and seems to be closely related to the subsequent developmental disorders such as schizophrenia and ASD (4, 5); There is an epidemiological association between exposure to influenza (H1N1 influenza) infection during pregnancy and an increased risk of developing neuropsychiatric disorders, suggesting that activation of the maternal immune system and an inflammatory response in the foetus (6) may be the origin of abnormal brain development (7).
The analysis of the gene expression of the offspring from mothers exposed to influenza showed the existence of an overexpression of genes associated with schizophrenia (8). However, the knowledge of the underlying pathogenic mechanisms and the associated molecular bases are not yet clear, attending that studies of this type in humans are very scarce and limited in their methodology. Several mechanisms have been proposed to explain the way in which maternal infection can interfere with brain development in offspring: (i) a systemic allostatic overload with loss of structural and functional integrity of the placenta; (ii) an activation of maternal and foetal immune responses with the production of neuronal antibodies and pro-inflammatory cytokines / chemokines and establishment of a pro-inflammatory state in the foetus and new-born; (iii) an interference in foetal neurodevelopment through the direct brain infection (6, 9).
Thanks to animal models, an even more robust immunological hypothesis has been outlined about the etiopathogenesis of various mental disorders and ASD. Some relevant neuroimmune factors found in the Central Nervous System (CNS) of murine models of maternal immunological activation (MIA) are: the activation of the microglia in a region-specific way (10), a decrease in the number of parvalbuminergic interneurons (11), alterations in the extracellular matrix (9) hyperactivity of innate immune pathways (12) changes in kynurenine metabolism (13) or alterations in cytokine levels, specifically interleukin (IL)-6, IL-10 and IL-1β, and tumour necrosis factors (TNF), specifically TNFα and TNFβ. However, not all stressed or immune challenged animals, develop behavioral alterations and animal models can explain differences between vulnerable or resilient phenotypes (14).
All these alterations have been suggested as possible biological mechanisms involved in neuropsychiatric disorders (15, 16). The innate immune response of CNS cells promotes and modulates the recruitment and activation of peripheral immune cells through chemokine expression, Blood-Brain Barrier (BBB) modulation, and cell-cell interaction. In this sense, the monocyte / macrophage axis plays a fundamental role with a direct correlation between the intracellular production of pro-inflammatory cytokines in monocytes and the levels of inflammatory biomarkers (17).
It is important to highlight the role that the placenta plays in possible neurodevelopmental alterations. In a recent publication entitled, “The placenta as a window to the brain” (18), the investigators review and highlight the role of placental markers in prenatal neurodevelopmental programming. In this line of work, cohorts of new-borns with Zika virus infection are a design model when it comes to detecting alterations in the neurodevelopment of these children and their biomarkers associated with viral infection (19). Currently, there are methods to obtain stem cells from umbilical cord blood that can be later differentiated into neurons (20). Another important contribution of this proposal is the possibility of generating a biobank of umbilical cord blood samples from pregnant women with COVID-19. The samples obtained in the study proposed in this project would allow, in the future, the generation of neural progenitor lines. That would give rise to in vitro neuronal cultures where the cellular and molecular alterations resulting from viral infection processes, as well as stress to which mothers have been subjected during pregnancy, could be explored. In this sense, in considering a cohort of pregnancies positive for SARS-CoV-2 and a negative uninfected cohort, the longitudinal study of births in these cases will allow an exploration of the hypothesis that relates infections (inflammatory alterations) during pregnancy, neurodevelopmental abnormalities, and an increased risk of neuropsychiatric diseases (21). Recent studies already have reported findings that suggest an immunological legacy imprinted on the neonate following maternal SARS-CoV-2 exposure, with potential far-reaching consequences (22–24).
A group of newborns of mothers infected by SARS-CoV-2 will present biological markers suggestive of presenting an abnormal inflammatory state (inflammation signature) that puts them at risk of suffering neurodevelopmental alterations. Inflammatory alterations in newborns will be related to clinical variables such as the time (gestational age) of maternal infection and its severity and, based on previous literature, alterations in the innate immune response will be associated with the clinical phenotype of the newborn.
Research hypothesis
All this information, with different degrees of evidence, leads us to take advantage of the exceptional situation produced by the COVID-19 pandemic, to try to answer the following questions: (i) To understand the inflammatory / immune status of the newborn (NB) of mothers infected by SARS-CoV-2?; (ii) is there a relationship between the clinical characteristics of the maternal infection (severity / time of infection / etc…) and the inflammatory status of the newborn?; (iii) could these features increase the vulnerability to develop CNS alterations at an early age, neurodevelopmental alterations during the first years of life and eventually during adult life?; (iv) How is the SARS-CoV-2 infected mother's placenta altered? Do the placental alterations COVID-19 mediated contribute to developing CNS alterations? (v) is the infection associated with phenotypes obtained from the neurological and neurodevelopmental clinical evaluation (hypotonia, clumsiness, impaired communication and sociability) in newborns, and at 6 and 12 months and 24 months?
Methods and analysis
Study design and data sources
A prospective study of cohorts of SARS-CoV-2 infected and uninfected pregnant women during the gestational period and of a cohort of newborns of these pregnancies, with a prospective follow-up of 24 months. Confirming to international standards for research ethics, this project was approved by the local institutional review board (the Clinical Research Ethics Committee of Hospital Virgen del Rocío). Patients meeting inclusion criteria provided written informed consent prior to their inclusion in the study.
Setting and procedures
The study will be carried out at the Hospital Universitario Virgen del Rocío (HUVR) and the Instituto de Biomedicina de Sevilla (IBiS). The multidisciplinary research team will include various specialists in pediatrics, psychiatry, gynaecology, clinical psychology, primary care, as well as scientists who are experts in basic and translational research, immunology and infectious diseases. The SARS Cov-2 uninfected women will be taken in primary care centres.
HUVR has a protocol for care for pregnant women in cases of COVID-19. All SARS-CoV-2 infected women attend Gynaecology consultations, specifically created (pregnancy and COVID-19 +). Once a case has been identified, a patient will be invited to participate, after confirming inclusion and exclusion criteria and signing the informed consent. In parallel, a SARS Cov-2 uninfected women with the same gestation time will be included. The same tests will be performed, plus a test that confirms non-infection by SARS-CoV-2 or previous COVID-19 infection. All ultrasound tests will be performed at the HUVR for homogenization. All pregnant women who acquire the COVID-19 and give birth during the inclusion period (1 of January of 2021 to 31 of august of 2022) and who are treated at the HUVR gynaecology and obstetrics service [pregnant (+) COVID-19] will be included. In addition, the mother / baby dyad will be followed up for 24 months postpartum.
The cohort was divided in two groups: 1,000 SARS-CoV-2 infected women and 1,000 SARS Cov-2 uninfected women. (Figure 1, left panel). Two subsample (Subsample A and B) (Figure 1, left panel) were chosen to adhere to our interest in the relationship between prenatal stress and anxiety, proinflammatory status during pregnancy and neurodevelopmental disorders (Figure 1, middle panel); All women from the cohort will be assessed, women and children from subsample A will be assessed with mental health questionnaires, using the M-CHAT and Bayley assessment, and subsample B will be additionally assessed biologically. All these information will be presented as working packages (WP) (see WP 3, 4, 6,) (Figure 1, middle and right panel).
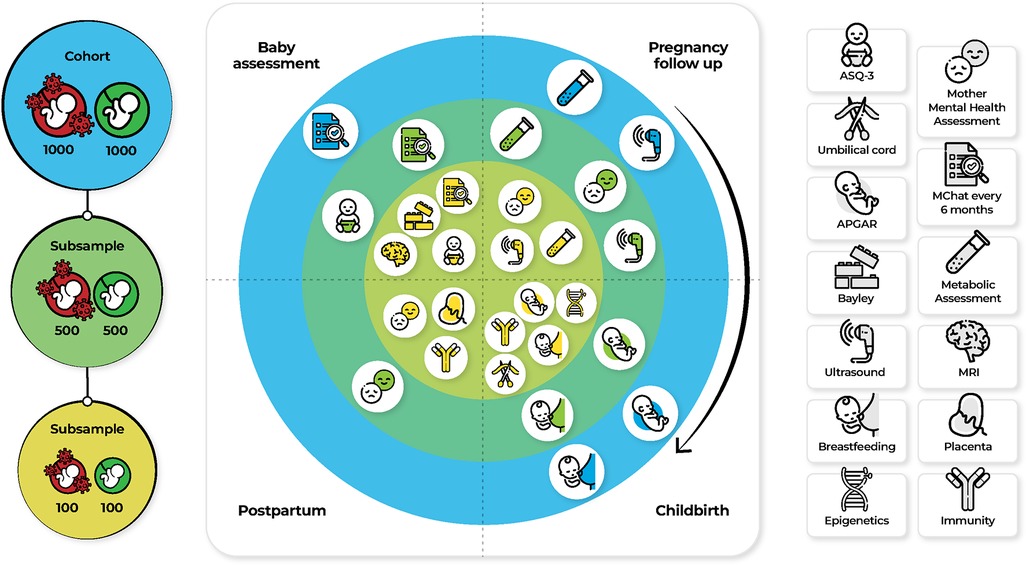
Figure 1. Assessment and measures for the specific aims in three samples. Abbreviations: APGAR, Appearance, Pulse, Grimace, Activity, and Respiration; ASQ-3, Ages and stages questionnaires; M-CHAT, Modified Checklist for Autism in Toddlers, Revised, with Follow-Up; MRI, Magnetic Resonance Imaging.
Study group
The SARS CoV-2 infected women cohort inclusion criteria are: (i) Pregnant and SARS CoV-2 infection; (ii) Over 18 years old; (iii) Ultrasound-confirmed pregnancy; (iv) Suffering COVID-19 (clinical criteria of a suspected case and with a positive PCR). (v) Asymptomatic person with a positive PCR with negative Immunoglobulin G (IgG)). The SARS CoV-2 infected women cohort exclusion criteria are: (i) Presents alcohol abuse during pregnancy; (ii) Other concomitant causes of risk of demonstrated neurodevelopmental disorders; (iii) Presents drug abuse except tobacco during pregnancy; (iv) under 18 years of age.
Control group
The SARS CoV-2 uninfected women cohort inclusion criteria are: (i) Over 18 years old; (ii) Ultrasound-confirmed pregnancy. The SARS Cov-2 uninfected women cohort exclusion criteria are: (i) Presents alcohol abuse during pregnancy; (ii) Other concomitant causes of risk of demonstrated neurodevelopmental disorders; (iii) presents drug abuse except tobacco during pregnancy; (iv) under 18 years of age. If during the pregnancy follow-up, a SARS Cov-2 uninfected women presents a SARS-CoV-2 infection, it would be assigned to the group of SARS CoV-2 infected women.
Sample size calculation
Sample size was calculated based on the reported data about the prevalence of neurodevelopmental disorders in children 58/100.000 (25). In order to obtain a difference of 3% between exposed and unexposed and according to the parameter choices for a desired power of 0.80% and 95% confidence level, we estimated that we would need 734 participants distributed over 367 SARS CoV-2 infected women and 367 SARS Cov-2 uninfected women for cohort A. Sample size analysis was conducted using Epidata software (26).
Statistics
The Kolmogorov–Smirnov test will be used to examine the normality of the data and the Levene test will be used to analyse the homogeneity of variances. If the distribution of the variables does not follow a normal law, non-parametric tests will be used for its analysis (W-Wilcoxon Signed-Rank test for paired and dependent data, U-Mann Whitney test for independent data). The association between the Bayle III score and pro-inflammatory marker measures will be made through Pearson or Spearman correlation analysis, as appropriate. The dichotomised score comparison of the neonatologist's evaluation (normal vs. abnormal) between groups (infected vs. uninfected) will be carried out by applying the Pearson χ2 test. To compare the presence of pro-inflammatory markers between the two groups (infected vs. uninfected), a comparative t-student or Mann-Whitney test will be performed, as appropriate. The presence of pro-inflammatory markers in the group of newborns of mothers infected by SARS-CoV-2 will be examined with a two-tailed hypothesis at a power of 80% and an α level of 5%. All the analyses will be performed with version 25.0 SPSS Inc. To explore the hypothesis that the biological alterations of interest play a causal role in the phenotypic alterations, mediation analyses will be performed exploring whether the inflammatory and immunological alterations (mediators collected at birth) mediate the relationship between exposure (SARS-CoV-2-infected mothers) and abnormal scores on the Essence questionnaire, ASQ-3 questionnaire, M-CHAT and Bayley III scale at 6 and 12 months.
Core variables and measures for the specific aims
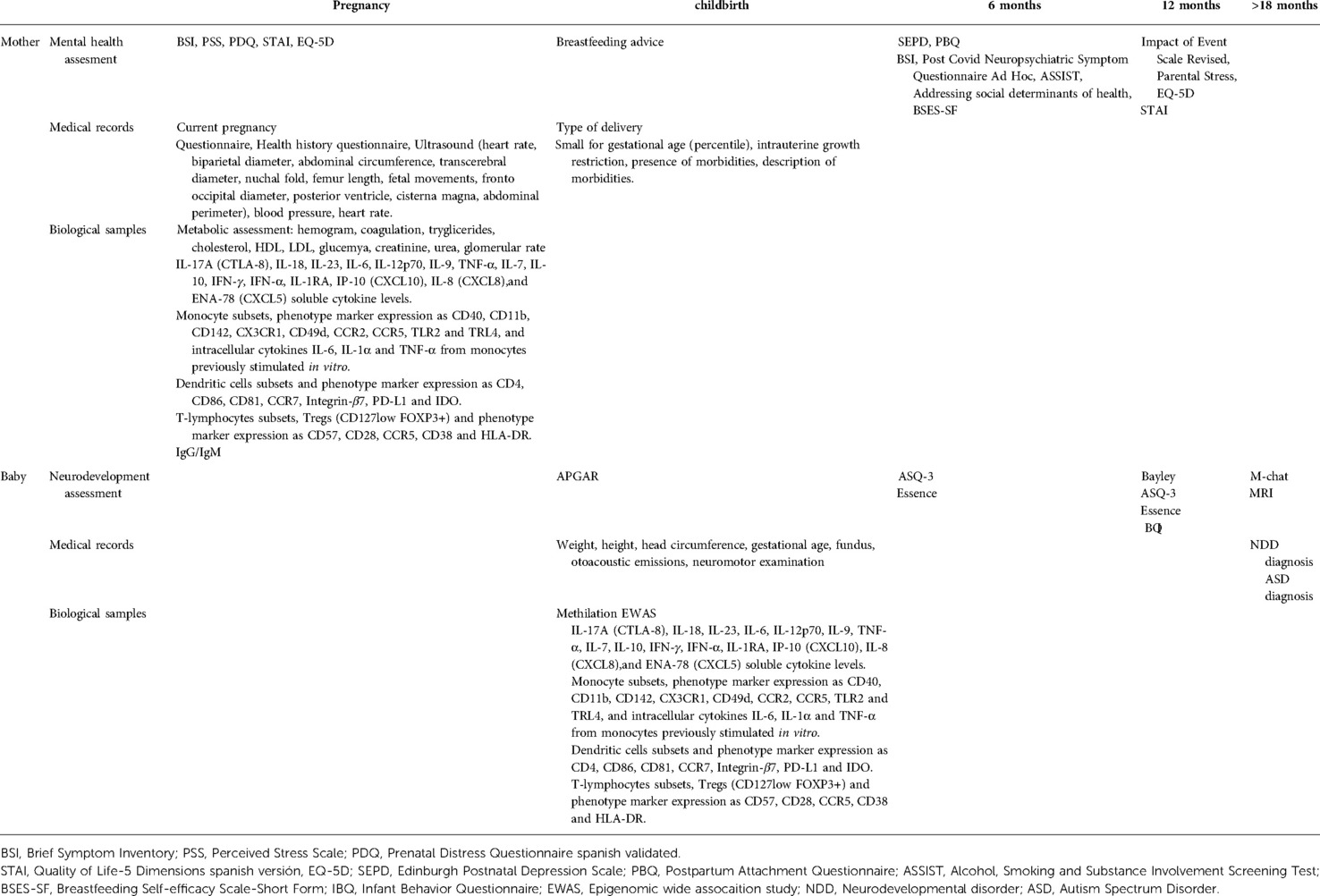
The main variable of the proposal will be the clinical and immunological characterisation of the newborn of (+) COVID-19 mothers, with neurological, neurodevelopmental, anthropometric, and immunological evaluations at birth, at 6 months and at 12 months, stratified by the week of pregnancy where the mother was infected by SARS-CoV-2. Table 1 In order to create two pregnant women clinical cohorts, the project is split into seven work packages (WP):
WP1: Pregnancy, birth and foetus assessment
Baseline data will be collected in relation to physical health history, pregnancy, ultrasound (heart rate, biparietal diameter, abdominal circumference, transcerebral diameter, nuchal fold, femur length, foetal movements, fronto occipital diameter, posterior ventricle, cisterna magna, abdominal perimeter), and biological samples, and a follow-up will be carried out every 3 months, which will coincide with those proposed by the protocol of the gynaecology service. The following variables will be measured: blood type, obstetric history (pre-eclampsia, threatened abortion, gestational diabetes, threatened preterm birth, intrauterine growth retardation, chorioamnionitis, eclampsia); blood pressure, heart rate, cholesterol, triglycerides, glycaemia, drugs consumption, history of maternal inflammatory diseases and vaccination in pregnancy. Additionally, during labour, the following data will be collected: induced childbirth, spontaneous, instrument-assisted vaginal delivery, placenta, breastfeeding advice, early breastfeeding, and complications of childbirth.
WP2: Mental health
Pregnant women and postnatal dyads (mothers and newborns) from cohort B will be evaluated through interviews conducted by the researcher. Scales and questionnaires will be applied with which the antecedents, social and psychological factors, and perceived stress during pregnancy will be exhaustively evaluated. Reactive psychosis cases have been reported as consequence of emotional stress related to SARS-CoV-2 infection (27). Brief Symptom Inventory (BSI) (28), State Trait Anxiety Inventory (STAI) (29), Perceived Stress Scale (PSS) (30), Prenatal Distress Questionnaire spanish validated (PDQ) (31), and Quality of Life-5 Dimensions spanish version (EQ-5D) (32) will be used. At 6 months, the following questionnaires will be performed: Edinburgh Postnatal Depression Scale (SEPD) (33), Postpartum Bonding Questionnaire (PBQ) (34), Brief Symptom Inventory (BSI) (28), Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) (‘The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): (35) / Addressing social determinants of health (36). Neuropsychiatric Symptoms will be assessed with Post-Covid Neuropsychiatric Symptom Questionnaire after 6 months of infection (37, 38) (Table 2). Additionally, at 12 months, stress will be measured using psychological questionnaires such as: Parental Stress Scale (PSI-SF) (39), Impact of Event Scale-Revises (IES-R) (40).
WP3: Inflammation
The main objective is to achieve: prognostic immunologic factors associated with cognitive impairment in children born of SARS-CoV-2-infected mothers. The integration of clinical and immunological data could elucidate immunological signatures influencing cognitive impairment in newborns of mothers infected with SARS-CoV-2. Cytokine quantification in plasma, a proinflammatory functional profile in monocytes, and a deep PBMC immunophenotyping (monocytes, dendritic cells and T-cells) using PBMCs from mothers with different COVID-19 symptomatology and their children will be evaluated. Firstly, IL-17A (CTLA-8), IL-18, IL-23, IL-6, IL-12p70, IL-9, TNF-α, IL-7, IL-10, IFN-γ, IFN α, IL-1RA, IP-10 (CXCL10), IL-8 (CXCL8), and ENA-78 (CXCL5) cytokines levels will be assayed in plasma samples utilising different kits according to the manufacturer's instructions.
Secondly, to proinflammatory functional profile in monocytes, PBMCs will be stimulated in vitro for 5 h at 37 °C with 0.5 μl/ml of lipopolysaccharide (LPS, Invivogen) in R-10 medium, including other condition with PBMCs without any stimulation as a negative control. Monensin (Golgi Stop, BD Biosciences) was added to all experimental conditions. Intracellular cytokines IL-6, IL-1α and TNF-α will be analysed by flow cytometry. Finally, PBMC immunophenotyping will be assayed. For monocyte immunophenotyping, PBMCs will be evaluated for viability using LIVE/DEAD Fixable Violet Dead Cell Stain Kit. CD3, CD19, CD20, CD56, CD14, CD16 and HLA-DR antibodies will be used to assess lineage. CD40, CD11b, CD142, CX3CR1, CD49d, CCR2 and CCR5 antibodies will be used to assess activation and cell adhesion surface markers. Toll-like receptor (TLR)-2 and TLR4 antibodies will be used to assess TLR surface expression. For dendritic cells immunostaining, PBMCs will be evaluated for viability using LIVE/DEAD fixable Aqua Blue Dead Cell Stain. Lin-22 (CD3, CD19, CD20, CD14 and CD56), HLA-DR, CD11c, CD16, CD123, CD1c, CD4 and CD141 antibodies will be used to evaluate lineage. CD86 and CD81 antibodies will be used to assess activation. CCR7 and Integrin-β7 antibodies will be used to evaluate cellular homing and PD-L1 and IDO will be used to assess intracellular expression of suppression markers. T lymphocyte immunophenotyping will include staining with LIVE/DEAD fixable Aqua Blue Dead Cell Stain for viability and antibodies against CD14, CD19, CD56, CD8 and CD3 to evaluate lineage and CD27 and CD45RA to determine their subsets pending on maturation. CD57, CD28, CCR5, CD38 and HLA-DR antibodies will be used to assess senescence and activation, and survival and FOXP3 and CD127 to assess regulatory T cells (Tregs). Multiparametric flow cytometry was performed on an LRS Fortessa flow cytometer using FACS Diva software (BD Biosciences). Data were analysed using the FlowJo 10.7.1 software (Treestar, Ashland, OR).
WP4: Placenta
The placenta is considered the main modulator of maternal-foetal interchange and plays a key role in foetal development (18). Furthermore, the placenta has intrinsic functions. For example, the serotonin synthesised by the placenta is necessary for foetal neurodevelopment, including processes such as cortical neurogenesis, migration and initial axon targeting (41). Disruption of the placental-mediated serotoninergic interchange may occur in prenatal maternal infection and/or stress causing impaired development and, thereby, contributing to neurodevelopmental disorders such as ASD and schizophrenia. Recently (42), have evidenced that placentas from women with SARS-CoV-2 display alterations in their immune collection, affecting the neonatal immune system. Their results indicate that SARS-CoV-2 infection differentially impacts the transcriptome of the mother and the neonate and that such changes are partly shared with those in the placental tissues. How these placental alterations impact in the foetal development and if the serotoninergic interchange is affected remain to be clarified and require further investigations. Accordingly, our research group is currently collecting placental samples, from control and SARS-CoV-2 infected pregnant women, in order to establish a biobank for our research goals. To achieve an optimal sampling, we are following the recommendations suggested in the collection protocol of placental samples from (43).
WP5: Neonatal assessment and neurodevelopment
The following data will be collected from medical records: sex (0, female; 1, male), gestational age (weeks), birth weight (grams), Apgar at minute 1 and 5 (range: 1–10), head circumference (centimetres), length (cm), type of delivery (0, spontaneous vaginal; 1, operative vaginal; 2, elective caesarean; 3, emergency caesarean), small for gestational age (percentile), intrauterine growth restriction (0, no; 1, yes), presence of morbidities (0, no; 1, yes), description of morbidities. The specific follow-up protocol will be similar in the most relevant aspects to the one used for the NB less than 1.500 grams (g) or less than 32 weeks of gestation age (Pallás Alonso et al., 2018) which includes: weight, height, head circumference, gestational age, fundus, otoacoustic emissions, and neuromotor examination. COVID-19 diagnosis will be performed by PCR / NB serum. At 6 months the following questionnaires will be performed by the parents: Essence Q Rev (44)./ Ages and stages questionnaires (ASQ-3) (45). At 12 months, ASQ-3 and the Bayley III scale (46) will be performed. Additionally we will collect data about breastfeeding duration (6 months and 12 months) and Breastfeeding Self-efficacy Scale-Short Form (BSES-SF) (47). At 18 months, Modified Checklist for Autism in Toddlers, Revised, with Follow-Up (M-CHAT) (48) questionnaires will be performed and ASD assessment by a specialist team when m-chat is positive (Table 1).
WP6: Infectiology
Identification of SARS-CoV-2 RNA will be performed in plasma samples of mothers (peripheral blood) and babies (umbilical cord blood) and, in the positive cases, the viral load will be determined. Identification of SARS-CoV-2 will be carried out in placenta samples, as well as the viral load. SARS-CoV-2 viability in blood from the mothers and babies and in placenta will be analysed in cellular cultures. In addition, the quantification of IgG/IgM antibodies will be evaluated in the serum samples of the patients (mothers and babies).
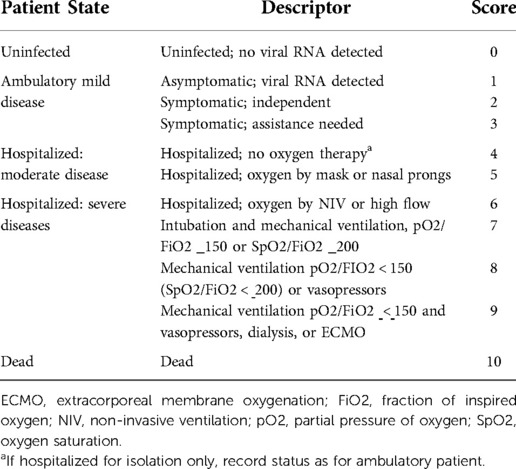
Placenta tissue samples will be assayed by electron microscopy to visualise the intracellular location of SARS-CoV-2 virions. In addition, immunohistochemical staining will be performed on formalin-fixed and paraffin-embedded tissue sections to evaluate the expression and distribution of the SARS-CoV-2 antigens (49). We will measure severity signs of COVID-19 during pregnancy, according to the WHO clinical progression scale (Table 3) (50).
WP7: Neuroimaging
Twenty 3- years old children from infected mothers group will be scanned with Magnetic Resonance Imaging (MRI) for establishing brain structural alterations. During a single session, two different sequences will be acquired: (i) Diffusion Tensor Image, which will allow establishing inflammatory and microstructural alterations of the white matter in areas associated with ASD (such as limbic-cortical pathway) and (ii) MPRAGE T1, standard sequence deriving morphometric measurements such as volume, cortical thickness and gyrification which has been also associated with ASD. MRI will be processed using established and validate pipelines implemented in Free surfer (https://surfer.nmr.mgh.harvard.edu/). As there will be no control group, we will use a strategy for data normalization that will exploit images of individuals in the same age group and gender collected in public data using the methodology described in Bethlehem et al., 2022 (51). Thanks to its online tool (https://brainchart.shinyapps.io/brainchart/) it will be possible effectively detect abnormal deviations of brain maturational trajectories of our cohort of 3-years old children.
WP8: Epigenetics
The genome methylation pattern of newborns will be determined from blood samples collected at the time of delivery. The samples will be outsourced and processed. To detect the methylation pattern, the Illumina Infinium Human MethylationEPIC BeadChip kit high-throughput array will be used. From the data obtained, differential methylation analysis will be performed with GenomeStudio and R software.
Results
This is a protocol report and no analyses have been conducted yet, being currently at, our study is in the recruitment process step. At the time of this publication, we have identified 1,060 SARS-CoV-2 infected mothers and all have already given birth. From the total of identified mothers, we have recruited 537 SARS-COV-2 infected women and all of them have completed the mental health assessment during pregnancy. We have collected biological samples from 119 mothers and babies. Only 214 babies are 12 months old, 385 babies are between 6 and 11 months old and 431 babies are younger than 6 months. Additionally, we have recruited 390 non-infected pregnant women.
Discussion
In this study, we will create two epidemiological cohorts (clinical and biological information) of pregnant women in the health area of the HUVR: a cohort of pregnant women (+) COVID-19 and a cohort of pregnant women (-) COVID-19 in which we will investigate the signature status of inflammation during pregnancy and in their offspring. In addition, we will evaluate: (i) the risk of developing psychosis or postpartum depression in mothers (+) COVID-19, we will identify signs and symptoms of neurodevelopmental disorders in neonates born to mothers (+) COVID, both at birth and at 6 and 12 months of life; (ii) we will analyse the relationship between the inflammatory signature (elevated inflammatory state) and developmental disorders or the appearance of neuropsychiatric disorders during the first years of life; (iii) we will obtain umbilical cord blood cells with stem cell phenotype with the potential to differentiate into neural lineages. (iv) we will establish a systematic follow-up protocol for the HUVR cohort of COVID-19 newborns as children at a high risk of developing neurodevelopmental disorders.
Expected results
Neurodevelopmental alterations will be evidenced by clinical signs in the first year of life: (i) difficulty directing their gaze in the direction in which another person is looking or pointing; (ii) a lack of joint attention: gaze does not alternate between an object and the adult showing or holding it; (iii) absence of communicative gestures; (iv) absence of socio-communicative babbling; (v) absence of social smile and spontaneous imitation; (vi) lack of interest in playing; (vii) lack of response when called by name; (viii) abnormal muscle tone, posture and movement patterns. H1. Analysis of biological samples from mothers and newborns will show changes in cytokine balance in T-helper Type 1 (TH1) cells (TNF-a, IFN-g) and TH2 cytokines (interleukins IL-4, IL-5, IL-6). More significant changes in the phenotype (monocyte function and activation of the umbilical cord sample) will be observed in the cases of pregnant women who were infected by SARS-CoV-2 in the first trimester of pregnancy. H2. Babies of mothers who were infected by SARS-CoV-2 will present high scores in the neurological evaluation (evaluating motor, language and motor function performed with the Bayley III scale) at 12 months. Regardless of the trimester in which they had the infection, they will present a greater alteration in neurodevelopment measured according to this scale and children will be considered at higher risk of developing a neuropsychiatric disorder in adulthood.
It has been reported that first and second trimester maternal SARS-CoV-2 infection is a risk factor for preterm birth and stillbirth (52, 53), and that birth during the pandemic, but not being infected by SARS-CoV-2, is associated with alterations in neurodevelopment at age 6 months (54). SARS-CoV-2 during late pregnancy did not increase the risk of developmental delay of the offspring 3 months after delivery. However, SARS-CoV-2 may have indirect effects on early childhood development by increasing mother-infant separation. The current worldwide research is to focus on exploring the role of target gene methylation levels in mediating the association between maternal prenatal stress related to the COVID-19 emergency and infant developmental outcomes (55) and generating knowledge about the psychological consequences of pandemics on pregnant individuals and to point toward prevention and intervention targets (56).
In utero exposure to infections have been shown to increase the risk of developing ASD or other neuropsychiatric diseases (1). Our proposal seeks to complete an innovative collaboration strategy between clinical and basic researchers that look toward interdisciplinary and integrative research to answer a research question that transcends the borders of the areas of knowledge involved individually and which is of high social importance. It is too soon to observe the consequences of in utero SARS-CoV-2 exposure on neurodevelopment; however, it is important to follow children exposed to SARS-CoV-2 in utero to determine the risk of the long-term neurodevelopmental outcomes.
In order to homogenise the procedures, we are publishing the protocol of the SIGNATURE project that started in January 2021. The main objective of publishing the protocol is to discuss with other research groups the methods, and sharing similar instruments of measure in order to compare results and obtain a large sample around the world of pregnant women infected by SARS-CoV-2 and their newborns.
Ethics statement
The studies involving human participants were reviewed and approved by The Clinical Research Ethics Committee of Seville. The patients/participants provided their written informed consent to participate in this study.
Author contributions
MRV, ALD, RAA, JCL, LF, BCF, NGT conceived the idea; MCR, NGT, MRV, LA, SGC designed the study; ERM, BDF, APG, SGC, LC, RRG designed the experiments; all signature group collected samples; all signature group participated in recruiting individuals; NGT, APG and SGC wrote the manuscript., LC, SGC, MCR, APG, BDF, LA,RRG, RAA, JCL, PO, ERM, MRV, ES contributed to manuscript revisions. All authors contributed to the article and approved the submitted version.
Funding
This work has received support from the Fundación Alicia Koplowitz to realize the epigenetic wide association study and to the clinical assessment to the children. This work has also received public support from the Consejería de Salud y Familias para la financiación de la investigación, desarrollo e innovación (i + d + i) biomédica y en ciencias de la salud en Andalucía (CSyF 2021 - FEDER). Grant Grant number PECOVID- 0195-2020. Convocatoria financiada con Fondo Europeo de Desarrollo Regional (FEDER) al 80% dentro del Programa Operativo de Andalucía FEDER 2014-2020. Andalucía se mueve con Europa. NG-T received payment under Rio Hortega contract CM20-00015 with the Carlos III Health Institute.
Acknowledgments
We kindly thank all clinical staff at the Virgen del Rocío Maternity Hospital for support to collect clinical records, samples and provide clinical care to patients. We are highly indebted to the participants for their cooperation in this study. We also kindly thank Livio Provenzi, Andrea Edlow and Michelle Freund for helpful discussions regarding their experience in pregnancy and SARS-Cov-2 projects.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tioleco N, Silberman AE, Stratigos K, Banerjee-Basu S, Spann MN, Whitaker AH, et al. Prenatal maternal infection and risk for autism in offspring: a meta-analysis. Autism Res. (2021) 14(6):1296–316. doi: 10.1002/aur.2499
2. Zimmer A, Youngblood A, Adnane A, Miller BJ, Goldsmith DR. Prenatal exposure to viral infection and neuropsychiatric disorders in offspring: a review of the literature and recommendations for the COVID-19 pandemic. Brain Behav Immun. (2021) 91:756–70. doi: 10.1016/j.bbi.2020.10.024
3. Lopez-Diaz A, Ayesa-Arriola R, Crespo-Facorro B, Ruiz-Veguilla M. COVID-19 Infection during pregnancy and risk of neurodevelopmental disorders in offspring: time for collaborative research. Biol Psychiatry. (2021) 89(5):e29–30. doi: 10.1016/j.biopsych.2020.09.011
4. Menninger KA. Influenza and schizophrenia. An analysis of post-influenzal “dementia precox,” as of 1918, and five years later further studies of the psychiatric aspects of influenza. 1926. Am J Psychiatry. (1994) 151(6 Suppl):182–7. doi: 10.1176/ajp.151.6.182
5. Torrey EF, Bartko JJ, Yolken RH. Toxoplasma gondii and other risk factors for schizophrenia: an update. Schizophr Bull. (2012) 38(3):642–7. doi: 10.1093/schbul/sbs043
6. Kępińska AP, Iyegbe CO, Vernon AC, Yolken R, Murray RM, Pollak TA. Schizophrenia and influenza at the centenary of the 1918–1919 spanish influenza pandemic: mechanisms of psychosis risk. Front Psychiatry. (2020) 11:72. doi: 10.3389/fpsyt.2020.00072
7. Khambadkone SG, Cordner ZA, Tamashiro KLK. Maternal stressors and the developmental origins of neuropsychiatric risk. Front Neuroendocrinol. (2020) 57:100834. doi: 10.1016/j.yfrne.2020.100834
8. Corvin A, Morris DW. Genome-wide association studies: findings at the major histocompatibility complex locus in psychosis. Biol Psychiatry. (2014) 75(4):276–83. doi: 10.1016/j.biopsych.2013.09.018
9. Khandaker GM, Zimbron J, Lewis G, Jones PB. Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol Med. (2013) 43(2):239–57. doi: 10.1017/S0033291712000736
10. Bates V, Maharjan A, Millar J, Bilkey DK, Ward RD. Spared motivational modulation of cognitive effort in a maternal immune activation model of schizophrenia risk. Behav Neurosci. (2018) 132(1):66–74. doi: 10.1037/bne0000230
11. Vasistha NA, Pardo-Navarro M, Gasthaus J, Weijers D, Müller MK, García-González D, et al. Maternal inflammation has a profound effect on cortical interneuron development in a stage and subtype-specific manner. Mol Psychiatry. (2020) 25(10):2313–29. doi: 10.1038/s41380-019-0539-5
12. MacDowell KS, Munarriz-Cuezva E, Caso JR, Madrigal JLM, Zabala A, Meana JJ, et al. Paliperidone reverts toll-like receptor 3 signaling pathway activation and cognitive deficits in a maternal immune activation mouse model of schizophrenia. Neuropharmacology. (2017) 116:196–207. doi: 10.1016/j.neuropharm.2016.12.025
13. MacDowell KS, Munarriz-Cuezva E, Meana JJ, Leza JC, Ortega JE. Paliperidone reversion of maternal immune activation-induced changes on brain serotonin and kynurenine pathways. Front Pharmacol. (2021) 12:682602. doi: 10.3389/fphar.2021.682602
14. Cattane N, Vernon AC, Borsini A, Scassellati C, Endres D, Capuron L, et al. Preclinical animal models of mental illnesses to translate findings from the bench to the bedside: molecular brain mechanisms and peripheral biomarkers associated to early life stress or immune challenges. Eur Neuropsychopharmacol. (2022) 58:55–79. doi: 10.1016/j.euroneuro.2022.02.002
15. McCutcheon RA, Krystal JH, Howes OD. Dopamine and glutamate in schizophrenia: biology, symptoms and treatment. World Psychiatry. (2020) 19(1):15–33. doi: 10.1002/wps.20693
16. Steullet P, Cabungcal JH, Monin A, Dwir D, O’Donnell P, Cuenod M, et al. Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: a “central hub” in schizophrenia pathophysiology? Schizophr Res. (2016) 176(1):41–51. doi: 10.1016/j.schres.2014.06.021
17. de Pablo-Bernal RS, Cañizares J, Rosado I, Galvá MI, Alvarez-Ríos AI, Carrillo-Vico A, et al. Monocyte phenotype and polyfunctionality are associated with elevated soluble inflammatory markers, cytomegalovirus infection, and functional and cognitive decline in elderly adults. J Gerontol A Biol Sci Med Sci. (2016) 71(5):610–8. doi: 10.1093/gerona/glv121
18. Shallie PD, Naicker T. The placenta as a window to the brain: a review on the role of placental markers in prenatal programming of neurodevelopment. Int J Dev Neurosci. (2019) 73:41–9. doi: 10.1016/j.ijdevneu.2019.01.003
19. Cranston JS, Tiene SF, Nielsen-Saines K, Vasconcelos Z, Pone MV, Pone S, et al. Association between antenatal exposure to Zika virus and anatomical and neurodevelopmental abnormalities in children. JAMA Netw Open. (2020) 3(7):e209303. doi: 10.1001/jamanetworkopen.2020.9303
20. McGuckin C, Jurga M, Ali H, Strbad M, Forraz N. Culture of embryonic-like stem cells from human umbilical cord blood and onward differentiation to neural cells in vitro. Nat Protoc. (2008) 3(6):1046–55. doi: 10.1038/nprot.2008.69
21. Parker SE, Lijewski VA, Janulewicz PA, Collett BR, Speltz ML, Werler MM. Upper respiratory infection during pregnancy and neurodevelopmental outcomes among offspring. Neurotoxicol Teratol. (2016) 57:54–9. doi: 10.1016/j.ntt.2016.06.007
22. Coler B, Adams Waldorf K. Maternal COVID-19 leaves a lasting immunological impression on the fetus. Nat Immunol. (2021) 22(12):1472–3. doi: 10.1038/s41590-021-01072-3
23. Conti MG, Terreri S, Piano Mortari E, Albano C, Natale F, Boscarino G, et al. Immune response of neonates born to mothers infected with SARS-CoV-2. JAMA Netw Open. (2021) 4(11):e2132563. doi: 10.1001/jamanetworkopen.2021.32563
24. Gee S, Chandiramani M, Seow J, Pollock E, Modestini C, Das A, et al. The legacy of maternal SARS-CoV-2 infection on the immunology of the neonate. Nat Immunol. (2021) 22(12):1490–502. doi: 10.1038/s41590-021-01049-2
25. Baxter AJ, Brugha TS, Erskine HE, Scheurer RW, Vos T, Scott JG. The epidemiology and global burden of autism spectrum disorders. Psychol Med. (2015) 45(3):601–13. doi: 10.1017/S003329171400172X
26. Lauritsen J, Bruus M. A comprehensive tool for validated entry and documentation of data. The EpiData Association. 2004.
27. Valdes-Florido MJ, Lopez-Diaz A, Palermo-Zeballos FJ, Martinez-Molina I, Martin-Gil VE, Crespo-Facorro B, et al. Reactive psychoses in the context of the COVID-19 pandemic: clinical perspectives from a case series. Rev Psiquiatr Salud Ment. (2020) 13(2):90–4. doi: 10.1016/j.rpsm.2020.04.009
28. Derogatis LR, Melisaratos N. The brief symptom inventory: an introductory report. Psychol Med. (1983) 13(3):595–605. doi: 10.1017/S0033291700048017
29. Skapinakis P. Spielberger state-trait anxiety inventory. In: Michalos AC, editors. Encyclopedia of quality of life and well-being research. Dordrecht: Springer Netherlands (2014). p. 6261–4. doi: 10.1007/978-94-007-0753-5_2825
30. Chan SF, La Greca AM. Perceived stress scale (PSS). In: Gellman MD, Turner JR, editors. Encyclopedia of behavioral medicine. New York, NY: Springer New York (2013). p. 1454–5. doi: 10.1007/978-1-4419-1005-9_773
31. Caparros-Gonzalez RA, Perra O, Alderdice F, Lynn F, Lobel M, García-García I, et al. Psychometric validation of the prenatal distress questionnaire (PDQ) in pregnant women in Spain. Women Health. (2019) 59(8):937–52. doi: 10.1080/03630242.2019.1584143
32. Badia X, Roset M, Montserrat S, Herdman M, Segura A. The spanish version of EuroQol: a description and its applications. European quality of life scale. Med Clin (Barc). (1999) 112(Suppl 1):79–85. PMID: 1061880410618804
33. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry. (1987) 150:782–6. doi: 10.1192/bjp.150.6.782
34. Brockington IF, Fraser C, Wilson D. The postpartum bonding questionnaire: a validation. Arch Womens Ment Health. (2006) 9(5):233–42. doi: 10.1007/s00737-006-0132-1
35. The alcohol, smoking and substance involvement screening test (ASSIST): development, reliability and feasibility. Addiction. (2002) 97(9):1183–94. doi: 10.1046/j.1360-0443.2002.00185.x
36. Schickedanz A, Hamity C, Rogers A, Sharp AL, Jackson A. Clinician experiences and attitudes regarding screening for social determinants of health in a large integrated health system. Med Care. (2019) 57[Suppl 6 Suppl 2(Suppl 6 2)]:S197–201. doi: 10.1097/MLR.0000000000001051
37. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. (2021) 11(1):16144. doi: 10.1038/s41598-021-95565-8
38. Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month Neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. (2021) 8(5):416–27. doi: 10.1016/S2215-0366(21)00084-5
39. Abidin R, Flens JR, Austin WG. The parenting stress Index. In: Archer RP, editor. Forensic uses of clinical assessment instruments. Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers (2006). p. 297–328.
40. Weiss DS. The impact of event scale: revised. In: Wilson JP, Tang CSk, editors, Cross-cultural assessment of psychological trauma and PTSD. New York, NY, US: Springer Science + Business Media (2007). p. 219–38. (International and cultural psychology.).
41. Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC, et al. A transient placental source of serotonin for the fetal forebrain. Nature. (2011) 472(7343):347–50. doi: 10.1038/nature09972
42. Garcia-Flores V, Romero R, Xu Y, Theis KR, Arenas-Hernandez M, Miller D, et al. Maternal-fetal immune responses in pregnant women infected with SARS-CoV-2. Nat Commun. (2022 ) 13(1):320. doi: 10.1038/s41467-021-27745-z
43. Burton GJ, Sebire NJ, Myatt L, Tannetta D, Wang YL, Sadovsky Y, et al. Optimising sample collection for placental research. Placenta. (2014) 35(1):9–22. doi: 10.1016/j.placenta.2013.11.005
44. Gillberg C, Fernell E, Minnis H. Early symptomatic syndromes eliciting neurodevelopmental clinical examinations. Sci World J. (2014) 2014:710570. doi: 10.1155/2013/710570
45. Squires J, Potter L, Bricker D. The ASQ user's guide for the ages & stages questionnaires: a parent-completed, child-monitoring system. Baltimore, MD, US: Paul H Brookes Publishing (1995). xvi, 156 p. (The ASQ user's guide for the Ages & Stages Questionnaires: A parent-completed, child-monitoring system).
46. Michalec D. Bayley scales of infant development: third edition. In: Goldstein S, Naglieri JA, editors. Encyclopedia of child behavior and development. Boston, MA: Springer US (2011). p. 215–215. Available from: https://doi.org/10.1007/978-0-387-79061-9_295.
47. Amini P, Omani-Samani R, Sepidarkish M, Almasi-Hashiani A, Hosseini M, Maroufizadeh S. The breastfeeding self-efficacy scale-short form (BSES-SF): a validation study in Iranian mothers. BMC Res Notes. (2019) 12(1):622. doi: 10.1186/s13104-019-4656-7
48. Robins DL, Casagrande K, Barton M, Chen CMA, Dumont-Mathieu T, Fein D. Validation of the modified checklist for autism in toddlers, revised with follow-up (M-CHAT-R/F). Pediatrics. (2014) 133(1):37–45. doi: 10.1542/peds.2013-1813
49. Qian Q, Fan L, Liu W, Li J, Yue J, Wang M, et al. Direct evidence of active SARS-CoV-2 replication in the intestine. Clin Infect Dis. (2021) 73(3):361–6. doi: 10.1093/cid/ciaa925
50. WHO Working Group on the Clinical Characterisation and Management of Covid-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. (2020) 20(8):e192–7. doi: 10.1016/S1473-3099(20)30483-7
51. Bethlehem RAI, Seidlitz J, White SR, Vogel JW, Anderson KM, Adamson C, et al. Brain charts for the human lifespan. Nature. (2022) 604(7906):525-33. doi: 10.1038/s41586-022-04554-y. Erratum in: Nature. (2022) 610(7931):E635388223
52. Piekos SN, Roper RT, Hwang YM, Sorensen T, Price ND, Hood L, et al. The effect of maternal SARS-CoV-2 infection timing on birth outcomes: a retrospective multicentre cohort study. Lancet Digit Health. (2022) 4(2):e95–104. doi: 10.1016/S2589-7500(21)00250-8
53. Wood ME, Delgado M, Jonsson Funk M. Understanding the effects of the COVID-19 pandemic on infant development—the preterm problem. JAMA Pediatr. (2022) 176(6):e215570–e215570. doi: 10.1001/jamapediatrics.2021.5570. Erratum in: JAMA Pediatr. (2022) 176(5):52834982102
54. Shuffrey LC, Firestein MR, Kyle MH, Fields A, Alcántara C, Amso D, et al. Association of birth during the COVID-19 pandemic with neurodevelopmental Status at 6 months in infants with and without in utero exposure to maternal SARS-CoV-2 infection. JAMA Pediatr. (2022) 176(6):e215563. doi: 10.1001/jamapediatrics.2021.5563
55. Provenzi L, Grumi S, Giorda R, Biasucci G, Bonini R, Cavallini A, et al. Measuring the outcomes of maternal COVID-19-related prenatal exposure (MOM-COPE): study protocol for a multicentric longitudinal project. BMJ Open. (2020) 10(12):e044585. doi: 10.1136/bmjopen-2020-044585
56. Giesbrecht GF, Rojas L, Patel S, Kuret V, MacKinnon AL, Tomfohr-Madsen L, et al. Fear of COVID-19, mental health, and pregnancy outcomes in the pregnancy during the COVID-19 pandemic study: fear of COVID-19 and pregnancy outcomes. J Affect Disord. (2022) 299:483–91. doi: 10.1016/j.jad.2021.12.057
Keywords: pregnancy, neurodevelomental disorders, COVI-19 pandemic, SARS-CoV-2, autism (ASD), maternal mental health
Citation: Garrido-Torres N, Cerrillos L, García Cerro S, Pérez Gómez A, Canal-Rivero M, de Felipe B, Alameda L, Marqués Rodríguez R, Anillo S, Praena J, Duque Sánchez C, Roca C, Paniagua M, López Díaz A, Romero-García R, Olbrich P, Puertas Albarracín Martín de Porres, Reguera Pozuelo P, Sosa IL, Moreno Dueñas MB, Pineda Cachero R, Zamudio Juan L, García Rumi V, Guerrero Benitez M, Figueroa R, Martín Rendón AM, Partida A, Rodríguez Cocho MI, Gallardo Trujillo C, Gallego Jiménez I, García Spencer S, Gómez Verdugo M, Bermejo Fernández C, Pérez Benito M, Castillo Reina RE, Cejudo López A, Sánchez Tomás C, Chacón Gamero MÁ, Rubio A, Moreno Mellado A, Ramos Herrero V, Starr E, González Fernández de Palacios M, García Victori E, Pavón Delgado A, Fernández Cuervo I, Arias Ruiz A, Menéndez Gil IE, Domínguez Gómez I, Coca Mendoza I, Ayesa-Arriola R, Fañanas L, Leza Juan C, Cisneros José M, Sánchez Céspedes J, Ruiz-Mateos E, Crespo-Facorro B and Ruiz-Veguilla M (2022) Examining the immune signatures of SARS-CoV-2 infection in pregnancy and the impact on neurodevelopment: Protocol of the SIGNATURE longitudinal study. Front. Pediatr. 10:899445. doi: 10.3389/fped.2022.899445
Received: 18 March 2022; Accepted: 26 October 2022;
Published: 21 December 2022.
Edited by:
Sabine Plancoulaine, INSERM U1153 Centre de Recherche Épidémiologie et Statistique, FranceReviewed by:
Mary Lynn Dell, Tulane University, United StatesSanchita Bhattacharya, University of California, United States
© 2022 Garrido-Torres, Cerrillos, García Cerro, Pérez Gómez, Canal-Rivero, de Felipe, Alameda, Marqués Rodríguez, Anillo, Praena, Duque Sánchez, Roca, Paniagua, López Díaz, Romero-García, Olbrich, Puertas Albarracín, Reguera Pozuelo, Sosa, Moreno Dueñas, Pineda Cachero, Zamudio Juan, García Rumi, Guerrero Benitez, Figueroa, Martín Rendón, Partida, Rodríguez Cocho, Gallardo Trujillo, Gallego Jiménez, García Spencer, Gómez Verdugo, Bermejo Fernández, Pérez Benito, Castillo Reina, Cejudo López, Sánchez Tomás, Chacón Gamero María Ángeles, Rubio, Moreno Mellado, Ramos Herrero, Starr, González Fernández de Palacios, García Victori, Pavón Delgado, Fernández Cuervo, Arias Ruiz, Menéndez Gil, Domínguez Gómez, Coca Mendoza, Ayesa-Arriola, Fañanas, Leza, Cisneros, Sánchez Céspedes, Ruiz-Mateos, Crespo-Facorro and Ruiz-Veguilla This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Crespo-Facorro benedicto.crespo.sspa@juntadeandalucia.es
Specialty Section: This article was submitted to Child and Adolescent Psychiatry, a section of the journal Frontiers in Pediatrics
 Nathalia Garrido-Torres
Nathalia Garrido-Torres Lucas Cerrillos5
Lucas Cerrillos5  Susana García Cerro
Susana García Cerro Alberto Pérez Gómez
Alberto Pérez Gómez Manuel Canal-Rivero
Manuel Canal-Rivero Luis Alameda
Luis Alameda Cristina Roca
Cristina Roca Alvaro López Díaz
Alvaro López Díaz Rafael Romero-García
Rafael Romero-García Peter Olbrich
Peter Olbrich Pablo Reguera Pozuelo
Pablo Reguera Pozuelo Mercedes Guerrero Benitez
Mercedes Guerrero Benitez Lourdes Fañanas
Lourdes Fañanas Juan C Leza
Juan C Leza José M Cisneros
José M Cisneros Javier Sánchez Céspedes
Javier Sánchez Céspedes Ezequiel Ruiz-Mateos
Ezequiel Ruiz-Mateos Benedicto Crespo-Facorro
Benedicto Crespo-Facorro