Corals in the Mesophotic Zone (40–115 m) at the Barrier Reef Complex From San Andrés Island (Southwestern Caribbean)
- 1Departamento de Ciencias Biológicas, Facultad de Ciencias, Laboratorio de Biología Molecular Marina-BIOMMAR, Universidad de los Andes, Bogotá, Colombia
- 2Departamento de Biología, Facultad de Ciencias, Universidad Nacional de Colombia, Sede Bogotá, Bogotá, Colombia
- 3Corporación para el Desarrollo Sostenible del Archipiélago De San Andrés, Providencia y Santa Catalina (CORALINA), San Andrés, Colombia
Background
Shallow reefs in the SeaFlower Biosphere Reserve, even at the remotest bank atolls, are showing a steady decline in coral cover overall health condition during the last 20 years (Sánchez et al., 2019b). Mesophotic Coral Ecosystems (MCEs), located between 30 and >150 m of water depth, may act as a refuge of coral populations due to favorable conditions in this less altered environment (Bongaerts et al., 2010). Particularly, in our study area, San Andres Island, populations of corals reaching the lower (>60 m) mesophotic zone, 40–90 m, such as Agaricia undata exhibit genetic connectivity throughout its depth range (Gonzalez-Zapata et al., 2018a), supporting this zone as a major reef-building coral refuge. It has been suggested that depending on the type of endosymbiont, corals can acclimatize to deeper depths (Ziegler et al., 2015), which was in fact observed in the bacterial population from A. undata in San Andrés Island (Gonzalez-Zapata et al., 2018a). In addition, many species of fish, corals, and other invertebrates from shallow reefs are also found in mesophotic reefs and it is proposed that these populations could contribute to the recovery of affected populations in shallower areas following a disturbance (Kramer et al., 2019).
There are potential new species of corals and associated species, including common shallow-water fauna, in mesophotic reefs (Luck et al., 2013; Petrescu et al., 2014), which urges studies surveying coral diversity at these depths. However, these reefs have been rarely explored below 60 m in water depth. The dataset presented in this study, provide the first exploration of the mesophotic zone (40–120 m deep) in an oceanic barrier reef complex (SeaFlower Biosphere Reserve), San Andrés Island, Southwestern Caribbean. The dataset presented here includes the collection information, and community composition of corals sensu lato (stony corals, hydrocorals, black corals, and octocorals). The ultimate goal was to contribute to the understanding of sensible and vulnerable environments, in the SeaFlower Biosphere reserve, in which San Andrés Island is immersed.
Data Collection
We concentrated the study in the fore-reef slope of San Andrés Island barrier reef complex near the location called “Trampa de Tortugas” or “Trampa Tortuga,” which offered a number of logistic advantages. The site bears a shelter to anchor the supporting boat despite its location on the fore-reef terrace. In addition, this site provides the only accessible glimpse of the oldest slope of the barrier-reef complex of San Andrés (Geister, 1975; Díaz et al., 1995; Diaz et al., 1996). We explored the reef using Close-Circuit Rebreather (CCR) (Megalodon, Inner Space Systems) and hypoxic trimix techniques (e.g., 11% Oxygen and 60% Helium) with complete bail-out support for each diver. At the site, we installed a mooring block at 24 m as a gas station that had high oxygen bail-outs (O2 96%) and from where we tied a 200 m long reel down to a depth of 114 m. The reel was used to safely explore down the site and to have an easy return to shallower waters. Seven dives were planned with a maximum of 20–30 min of bottom time and the longest dives spanned 133–328 min including decompression stops. The sampling included digital imagery (NikonTM D7000, Nikkor micro 60 mm lens, Sea & SeaTM YS-D1 strobe, and AquaticaTM AD7000 housing) and 113 voucher specimens (dry and ethanol 96%), which were deposited at Museo de Historia ANDES (Bogotá, Colombia) (Table 1).
Coral Identifications
All specimens were examined under the optical and/or compound microscope for morphological identification and contrasted against species keys (if available) and/or taxonomic descriptions. For scleractinian corals we used Cairns, 2000; for Stylasteridae Cairns et al., 1986; for black corals Opresko and Sánchez, 2005; for octocorals Bayer, 1961; Bayer and Grasshoff, 1994, 1995; Sánchez and Wirshing, 2005; Sánchez, 2009. In addition, several species accounts for Colombia were also useful in species identification (Flórez et al., 2010; Chacón-Gómez et al., 2012; Santodomingo et al., 2013). When needed, Scanning Electron Microscopy (SEM) images were obtained at the microscopy laboratory in the Universidad de los Andes to increase the certainty of identifications.
Dataset Outcomes and Discussion
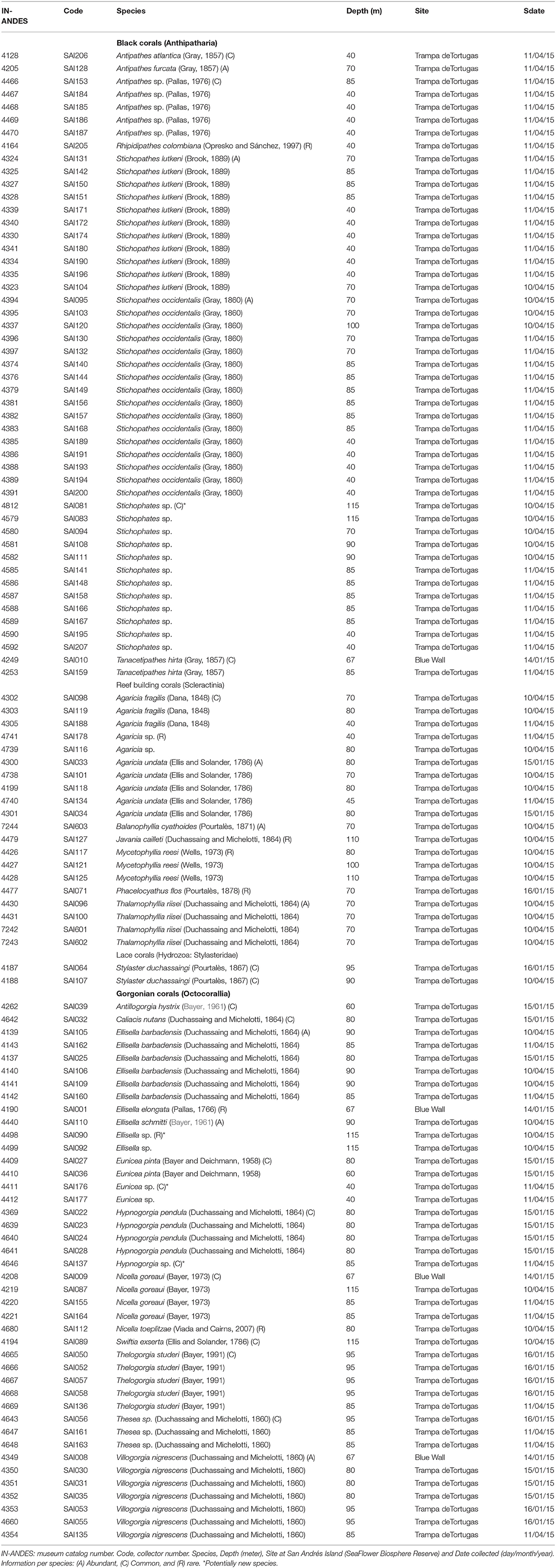
The dataset included 113 specimens from 33 species collected below 40 m (8 black corals, 1 lace coral, 8 scleractinian corals, and 16 gorgonian corals: Table 1). Exploring “Trampa de Tortugas,” we noticed the disappearance of most reef-building corals and zooxanthellate octocorals at different depths. Some reef-building corals, notably Mycethophyllia reesi, Agaricia undata, A. fragilis, and Madracis sp., distributed well into the lower mesophotic zone (~90 m) and are characterized by an increase in the presence of the euendolithic algae Ostreobium, which is clearly observable at the colony surface (Gonzalez-Zapata et al., 2018b). These colonial corals are replaced below 80 m by azooxanthellate cup corals, including Javania cailleti, Phacelocyathus flos, Balanophyllia cyathoides, and Thalamophyllia riisei forma solida (Figure 1). A noteworthy observation was the presence of Ostreobium at the basal portion of T. riisei cup-corals.
Figure 1. Selected corals in the lower mesophotic zone (45–70 m) at San Andrés Island (SeaFlower Biosphere reserve). (A–H) Black corals. (A) Antipathes furcata, (B,C) Antipathes atlantica, (D,E) Stichopathes spp. (F) Stichopathes lutkeni; (G) S. occidentalis; (H) Stichopathes sp. (probably undescribed species or not reported yet for the Caribbean) (scales F–H scale 200 and 50 μm for details). (I–K) Stylaster duchassaingi Pourtalès, 1867 (I,J). Colonies at Trampa Tortuga, 90 m. (K) Scanning Electron Microscopy (SEM) images detail of terminal branches (scale 500 μm). (L) Cup coral Thalamophyllia riisei forma solida, voucher samples from Trampa Tortuga, San Andrés, optical, and SEM, including a costae detail. (M) Cup corals Javania cailleti (left) (N) Balanophyllia cyathoides (right), voucher specimen from Trampa Tortuga, San Andrés. (O) Cup coral Phacelocyathus flos, voucher from Trampa Tortuga, San Andrés. Corals at 55 m and voucher specimen.
The lower mesophotic reef is also the habitat of many unique black corals (e.g., Rhipidipathes colombiana and Tanacetipathes hirta) and hydrocorals (Stylaster duchassaingi) but the most abundant group are azoxanthellate octocorals mostly from the Plexauridae family (Sánchez, 2017; Sánchez et al., 2019a). The species replacement is enhanced by short terraces intertwined with abrupt steps at every 10 m starting at 60, 90, 100, and 115 m, at “Trampa Tortuga” reef in San Andrés Island. On the leeward side of the Island, there is parallel slope sand corridor (mostly from Halimeda copiosa flocks) following the reef slope, which end at about 60–80, where the reef growth continues. The deepest zooxanthellate gorgonian coral was Antillogorgia hystrix (65 m), followed by Eunicea pinta (55 m). Occasionally, Muricea laxa, A. bipinnata, and A. americana were seen at depths of about 40 m. These zooxanthellate octocorals share habitat with some azooxanthellate octocorals such as Iciligorgia schrammi and diverse ellisellids (Sánchez et al., 2019a).
Black corals (Antipatharia) are also common from 30 m down to the lower mesophotic area (Bo et al., 2019). The most abundant black corals are Antipathes furcata (Figure 1A), A. caribbeana, A. atlantica (Figure 1B), Plumapathes pennacea, Stichopathes lutkeni, and S. occidentalis. Wire corals, Stichopathes spp., with colonies reaching more than 2 m long were seen in high densities of to have more than 10 colonies per square meter (Figures 1C,D). Below 70 m, the aforementioned black corals are less seen and other species emerge such as Rhipidipathes colombiana, first seen off the Colombian coast (Opresko and Sánchez, 2005), and Tanacetipathes hirta. There is also a great amount of wire corals from species we could not identify and probably comprise new undescribed species. Despite the clear characters of S. lutkeni and S. occidentalis under the electron microscope, there were specimens, Stichopathes sp., with conspicuously smaller spines not found in any other species described for this region (Figure 1H).
The most unexpected finding comprised a number of new records for several deep-sea corals, which have been usually found on deeper waters. Stylaster duchassaingi Pourtalès, 1867, a hydrocoral (Stylasteridae) was observed from 80 to 115 m forming seafan colonies up to 40 cm in height (Figures 1I–K). This is the southernmost record of the species and one of the shallower observations in its range. Stylaster roseus is commonly observed in the same reef but above 40 m (JAS, personal observation). San Andrés Island is the only coral reef complex so far in the Caribbean with two documented species of Stylaster.
As expected, the exploration of the lower mesophotic zone uncovered a great amount of new species records and potential discoveries (e.g., Stichopathes sp.). In addition, this is the first time that many of the species have been ever seen and photographed in their natural environment (Sánchez et al., 2019a). Continuing research in this environment will enrich the ecology, systematics, and conservation of understudied corals such as cup corals. For instance, the Thallamophyllia riisei cup coral found in San Andrés is extremely different to the reported T. riisei from the Colombian coast (Flórez et al., 2010), which is colonial with great differences in morphological traits. It is worth mention that this is the product of only seven dives (and <140 min of total bottom time) for San Andres Island.
Reuse Potential
The specimens collected and properly curated (deposited and IN-ANDES in Bogota, Colombia) comprise a valuable resource for further systematic studies in several groups of corals, which could comprise new species. In addition, it is important to mention that the specimens in this data report have not been monitored in the past, giving the logistic constraints of deep-sea diving. As the interest in MCEs increases biodiversity data becomes crucial for comparisons.
Data Availability
The datasets for this study can be found at https://ipt.biodiversidad.co/cr-sib/resource.do?r=0359_mesofoticos_20190729, titled “Biodiversidad y Conectividad de los arrecifes mesofóticos (30–120 m) de la costa Caribe colombiana”. The data presented here corresponds to coral specimens (Cnidaria: Anthozoa andHydrozoa) collected between 40 and 115 m in the mesophotic corals ecosystems from San Andrés Island (SeaFlower Biosphere Reserve).
Author Contributions
JS, LD, JA, and NB conceived the study. JS, JA, and NB collected the data. FG-Z, AS, DV, AP-V, and JS identified and processed the material. JS wrote the report with the help of LD, FG-Z, and NB. All authors read and accepted the manuscript.
Funding
This work was supported by an agreement between Corporación para el Desarrollo Sostenible del Departamento Archipiélago de San Andrés Providencia y Santa Catalina, CORALINA-Universidad de los Andes (Convenios No. 13, 2014 and No. 21, 2015: Protección y conservación de los recursos de la biodiversidad y de los ecosistemas estratégicos dentro de la Reserva de Biosfera Seaflower Fondo de Compensación Ambiental FCA del Ministerio de Ambiente y Desarrollo Sostenible). Additional funding was possible thanks to the University of Los Andes (Vicerrectoría de Investigaciones and Facultad de Ciencias) and COLCIENCIAS (Project code 120465944147).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The support from Bluelife dive shop (family Garcia) was fundamental to accomplish this study. The San Andres Hospital kindly supplied medical oxygen for CCR. We are very grateful with Gregg Stanton, Wakulla Dive Center, for continuing support and advise for deep diving. We are thankful with Fabian García, Santiago Herrera, Mariana Gnecco, Manu Forero, Federico Botero, and Camilo Martinez for fieldwork support. We recognize the participation and support from local communities.
References
Bayer, F. M. (1961). The Shallow Water Octocorallia of the West Indian Region. The Hague: Martinus Nijoff.
Bayer, F. M., and Grasshoff, M. (1994). The genus group taxa of the family Ellisellidae, with clarification of the genera established by JE Gray (Cnidaria: Octocorallia). Senckenb. Biol. 74, 21–45.
Bayer, F. M., and Grasshoff, M. (1995). Two new species of the gorgonacean genus Ctenocella (Coelenterata: Anthozoa, Octocorallia) from deep reefs in the western Atlantic. Bull. Mar. Sci. 56, 625–652.
Bo, M., Montgomery, A. D., Opresko, D. M., Wagner, D., and Bavestrello, G. (2019). “Antipatharians of the mesophotic zone: four case studies,” in Mesophotic Coral Ecosystems, Coral Reefs of the World, eds Y. Loya, K. A. Puglise, and T. C. L. Bridge (Cham: Springer Nature Switzerland AG, 683–708.
Bongaerts, P., Ridgway, T., Sampayo, E. M., and Hoegh-Guldberg, O. (2010). Assessing the ‘deep reef refugia' hypothesis: focus on Caribbean reefs. Coral Reefs 29, 309–327. doi: 10.1007/s00338-009-0581-x
Cairns, S. D. (2000). A revision of the shallow-water Azooxanthellate Scleractinia of the western Atlantic. Revisión de los corales azooxantelados (Scleractinia) de las aguas someras del Atlántico occidental. Stud. Fauna Curacao Caribbean Islands 75, 1–231.
Cairns, S. D., Cairns, S. D., Cairns, S. D., and Cairns, S. D. (1986). A Revision of the Northwest Atlantic Stylasteridae (Coelenterata: Hydrozoa). Washington, DC: Smithsonian Institution Press.
Chacón-Gómez, I. C., Reyes, J., and Santodomingo, N. (2012). Deep-water octocorals (Anthozoa: Cnidaria) collected from the Colombian Caribbean during'Macrofauna Explorations'1998-2002*. Bol. Investig. Mar. Costeras-INVEMAR, 41, 193–211.
Diaz, J. M., Diaz, G., Garzon-Ferreira, J., Geister, J., Sánchez, J. A., and Zea, S. (1996). Atlas de los arrecifes coralinos del Caribe colombiano. I. Archipiélago de San Andrés y Providencia. Publicaciones Especiales del INVEMAR, Santa Marta.
Díaz, J. M., Garzón-Ferreira, J., and Zea, S. (1995). Los arrecifes coralinos de la Isla de San Andrés, Colombia: estado actual y perspectivas para su conservación. Colecc. Jorge Alvarez Lleras, Academia Colombiana de Ciencias Exactas, Físicas y Naturales, 7.
Flórez, P., Reyes, J., and Santodomingo, N. (2010). Corales Escleractinios de Colombia. Instituto de Investigaciones Marinas y Costeras-INVEMAR.
Geister, J. (1975). Riffbau und geologische Entwicklungsgeschichte der Insel San Andres (westliches Karibisches meer. Kolurnbien). Stuttg. Beitr Naturk 15, 1–203.
Gonzalez-Zapata, F. L., Bongaerts, P., Ramírez-Portilla, C., Adu-Oppong, B., Walljasper, G., Reyes, A., et al. (2018a). Holobiont diversity in a reef-building coral over its entire depth range in the Mesophotic zone. Front. Mar. Sci. 5:29. doi: 10.3389/fmars.2018.00029
Gonzalez-Zapata, F. L., Gómez-Osorio, S., and Sánchez, J. A. (2018b). Conspicuous endolithic algal associations in a mesophotic reef-building coral. Coral Reefs 37, 705–709. doi: 10.1007/s00338-018-1695-9
Kramer, N., Eyal, G., Tamir, R., and Loya, Y. (2019). Upper mesophotic depths in the coral reefs of Eilat, Red Sea, offer suitable refuge grounds for coral settlement. Sci. Rep. 9:2263. doi: 10.1038/s41598-019-38795-1
Luck, D. G., Forsman, Z. H., Toonen, R. J., Leicht, S. J., and Kahng, S. E. (2013). Polyphyly and hidden species among Hawai'i's dominant mesophotic coral genera, Leptoseris and Pavona (Scleractinia: Agariciidae). PeerJ 1:e132. doi: 10.7717/peerj.132
Opresko, D. M., and Sánchez, J. A. (2005). Caribbean shallow-water black corals (Cnidaria: Anthozoa: Anthipatharia). Caribb. J. Sci. 41, 492–507.
Petrescu, I., Chatterjee, T., and Schizas, N. V. (2014). New species of Cumella (Crustacea: Cumacea: Nannastacidae) from mesophotic habitats of Mona Island, Puerto Rico, Caribbean Sea. Cah. Biol. Mar. 55, 183–189. doi: 10.11646/zootaxa.3476.1.2
Sánchez, J. A. (2009). Systematics of the candelabrum gorgonian corals (Eunicea Lamouroux; Plexauridae; Octocorallia; Cnidaria). Zool. J. Linn. Soc. 157, 237–263. doi: 10.1111/j.1096-3642.2008.00515.x
Sánchez, J. A. (2017). “Diversity and evolution of octocoral animal forests at both sides of tropical America,” in Marine Animal Forests, eds S. Rossi, L. Bramanti, A. Gori, and C. Orejas Saco del Valle (Cham: Springer, 111–143.
Sánchez, J. A., Dueñas, L. F., Rowley, S. J., González, F. L., Vergara, D. C., Montaño-Salazar, S. M., et al. (2019a). “Gorgonian corals (39),” in Mesophotic Coral Ecosystems, Coral Reefs of the World, eds Y. Loya, K. A. Puglise, and T. C. L. Bridge (Cham: Springer Nature Switzerland AG, 727–745.
Sánchez, J. A., Gómez-Corrales, M., Gutierrez-Cala, L., Vergara, D. C., Roa, P., González-Zapata, F. L., et al. (2019b). Steady decline of corals and other benthic organisms in the seaflower biosphere reserve (Southwestern Caribbean). Front. Mar. Sci. 6:73. doi: 10.3389/fmars.2019.00073
Sánchez, J. A., and Wirshing, H. H. (2005). A field key to the identification of tropical western Atlantic zooxanthellate octocorals (Octocorallia: Cnidaria). Caribb. J. Sci. 41, 508–522.
Santodomingo, N., Reyes, J., Flórez, P., Chacón-Gómez, I. C., van Ofwegen, L. P., and Hoeksema, B. W. (2013). Diversity and distribution of azooxanthellate corals in the Colombian Caribbean. Mar. Biodivers. 43, 7–22. doi: 10.1007/s12526-012-0131-6
Keywords: Mesophotic Coral Ecosystem (MCE), coral, octocoral, black coral, stylaster, scleractinia, San Andrés Island, Caribbean
Citation: Sánchez JA, González-Zapata FL, Dueñas LF, Andrade J, Pico-Vargas AL, Vergara DC, Sarmiento A and Bolaños N (2019) Corals in the Mesophotic Zone (40–115m) at the Barrier Reef Complex From San Andrés Island (Southwestern Caribbean). Front. Mar. Sci. 6:536. doi: 10.3389/fmars.2019.00536
Received: 05 June 2019; Accepted: 15 August 2019;
Published: 06 September 2019.
Edited by:
Zhijun Dong, Yantai Institute of Coastal Zone Research (CAS), ChinaReviewed by:
Xiubao Li, Hainan University, ChinaCarolina Bastidas, Massachusetts Institute of Technology, United States
Copyright © 2019 Sánchez, González-Zapata, Dueñas, Andrade, Pico-Vargas, Vergara, Sarmiento and Bolaños. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Armando Sánchez, juansanc@uniandes.edu.co
 Juan Armando Sánchez
Juan Armando Sánchez Fanny L. González-Zapata
Fanny L. González-Zapata Luisa F. Dueñas
Luisa F. Dueñas Julio Andrade
Julio Andrade Ana Lucía Pico-Vargas
Ana Lucía Pico-Vargas Diana Carolina Vergara
Diana Carolina Vergara Adriana Sarmiento
Adriana Sarmiento Nacor Bolaños3
Nacor Bolaños3