Subclinical Leaflets Thrombosis After Transcatheter Replacement of Bicuspid vs. Tricuspid Aortic Valve
- 1Department of Cardiology, Second Affiliated Hospital Zhejiang University School of Medicine, Hangzhou, China
- 2Zhejiang University School of Medicine, Hangzhou, China
Background: Subclinical leaflet thrombosis (SLT) is an important sequela that compromises the durability of the bioprosthetic valve.
Objectives: To better determine the effect of SLT in bicuspid aortic valve (BAV), we performed a retrospective assessment of CT-defined SLT in BAV and tricuspid aortic valve (TAV) stenotic patients.
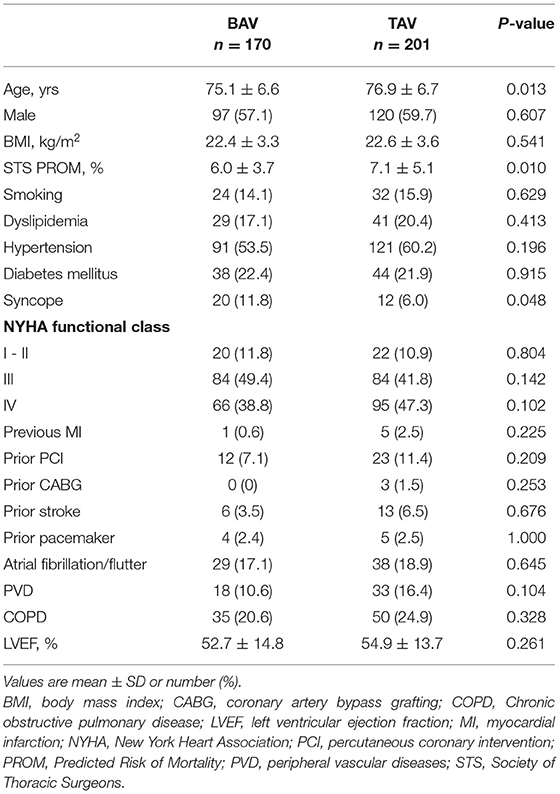
Methods: We consecutively collected patients undergoing the TAVR between August 2015 and March 2020 in our center. A total of 170 BAV and 201 TAV cases were enrolled. Multidetector computed tomography was performed within 30 days and at 1-year.
Results: Twenty cases in the BAV group and 19 cases in the TAV group had hypoattenuated leaflet thickening (HALT) in 30 days (12.5 vs. 9.9%, p = 0.449), and 52 cases in BAV and 61 cases in TAV had the HALT (34.9 vs. 36.7%, p = 0.733) at 1-year follow-up. The mean aortic gradient (MAG) and effective orifice areas (EOA) values were comparable between the two groups at 30 days (HALT vs. no HALT; 10.8 ± 4.8 vs. 11.3 ± 6.0, p = 0.638; 1.6 ± 0.4 vs. 1.6 ± 0.3, p = 0.724), and still, no difference was observed in the MAG at 1-year (11.5 ± 5.6 vs. 10.6 ± 5.1, p = 0.164). However, the EOA at 1-year was statistically different between the two groups (1.5 ± 0.3 vs. 1.6 ± 0.4, p = 0.004). The multivariate logistic regression analysis demonstrated the anticoagulation and age as independent predictors both in the BAV and TAV groups at 1-year. There was no difference in clinical events between the HALT and no HALT group in relevant to BAV or TAV at 1-year follow-up.
Conclusions: The presence of subclinical leaflet thrombosis defined by the CT was comparable between the BAV and TAV in the first year after the TAVR procedure. Age and anticoagulation were the independent predictors of the subclinical leaflet thrombosis at 1 year after the TAVR. There was no difference in relevant clinical events between the BAV and TAV groups at 1-year follow-up.
Introduction
In elderly patients with symptomatic aortic stenosis (AS), transcatheter aortic valve replacement (TAVR) is a less invasive heart procedure to replace the stenotic valve with a favorable prognosis. As the use of the TAVR for the indication of the AS expands to younger and low-risk patients, the goal of developing the durable bioprosthetic valve has been particularly focused on. Subclinical leaflet thrombosis (SLT) is a critical occurrence that jeopardizes the durability of the bioprosthetic valve. Hypoattenuated leaflet thickening (HALT) and reduced leaflet motion (RELM), as detected by multidetector computed tomography (MDCT) were hallmarks of the subclinical leaflet thrombosis (1–4). The occurrence of the SLT in transcatheter valve replacements is about 10–40% (3–7).
Due to severe and asymmetric calcification in the native aortic valves and the deformation of the bioprosthetic frames after the TAVR, the SLT in the bicuspid aortic valve (BAV) is very concerned (8). At present, there is no study to compare the SLT viewed by computed tomography (CT) and its clinical sequelae and prognosis between the BAV and tricuspid aortic valve (TAV). To better explore the SLT in the BAV, this study aimed to retrospectively assess the SLT defined by the CT in the BAV and TAV stenotic patients.
Methods
Study Population
This study was a retrospective observational analysis. We consecutively collected patients undergoing the TAVR between August 2015 and March 2020 in our center. Exclusion criteria: (1) Cases lacking the pre-procedure CT to define aortic valve type including quadricuspid valve; (2) Patients received bioprosthetic implant before the TAVR procedure; (3) Patients with contrast agents contraindicated, allergies, and severe renal dysfunction (estimated glomerular filtration rate of ≤ 30 ml/min); (4) Patients lost to follow up; (5) Patients with incomplete or inconclusive CT series.
The morphological type of aortic valve was classified into BAV (including type 0, type 1, and type 2) or TAV according to the Sievers classification (9). The study was approved by the local Ethical Committee and was in accordance with the principles of the Declaration of Helsinki.
TAVR Procedure and Antithrombotic Regimen
The TAVR procedures were performed in a hybrid operating room. Unfractionated heparin was used (50–70 U/kg) to maintain an activated clotting time (ACT) of >250 s during all procedures. Adopting general anesthesia or local anesthesia with sedation was decided by anesthetists. Transfemoral or non-transfemoral access was used based on the pre-procedure assessment. The majority of cases were implanted with self-expanding valves, and the rest of the patients were implanted with balloon-expandable or mechanically expanding valves. Post-dilatation was employed based on surgeons' discretion. A large proportion of the patients were prescribed dual antiplatelet therapy (DAPT) following the procedures. Oral anticoagulants (OAC) were recommended if the patients had indications of anticoagulation.
Echocardiography and Laboratory Tests
Transthoracic echocardiography (TTE) was performed before the TAVR procedure, before discharge, and at 30-day and 1-year follow-up. The mean aortic gradient (MAG), effective orifice area (EOA), left ventricular end-diastolic diameter (LVEDd), left atrium diameter, left ventricular ejection fraction (LVEF), and pulmonary arterial systolic pressure (PASP) were measured by the TTE. The results of the TTE were analyzed by experienced echocardiographers. The levels of the D-dimer and N-terminal pro-brain natriuretic peptide (NT-pro-BNP) were tested at each follow-up visit.
MDCT Acquisition and Analysis
Cardiac contrast-enhanced ECG-gated multidetector computed tomography (MDCT) was performed using Philips Brilliance iCT 256 (Philips Corporation, Amsterdam, Netherlands) or GE revolution CT (GE Healthcare, Chicago, IL, USA) with collimation of 0.6 or 0.8 mm, 100 or 120 kV for imaging.
Patients routinely underwent MDCT scanning before the procedure, before discharge or at 30 days after implantation (first CT) and at 1-year follow-up (second CT). Full phase CT imaging was acquired and analyzed by using 3mensio workstation (Pie Medical Imaging, Maastricht, Netherlands). Two authors (Dao Zhou and Hanyi Dai) evaluated the CT scans independently and one author (Gangjie Zhu) reviewed the data.
HALT and RELM
The HALT was evaluated in cardiac diastole. The area and thickness of hypoattenuation were measured in a cross-sectional 2D multiplanar reconstruction (MPR) view and corresponding 2D longitudinal MPR view, respectively. If the HALT was found, the RELM had been evaluated in cardiac systole with the 3D or 4D CT. According to the severity of the leaflet reduced motion, the RELM was graded as mild (<50%), moderate (≥50%, <70%), and severe (≥70%) (10). The moderate and severe RELM were denoted as hypoattenuation affecting motion (HAM) (10).
%RELM = (width of hypoattenuation/ (1/2 diameter of the bioprosthesis in the section)·100%.
Follow-Up and Clinical Adverse Events
Despite this study was a retrospective analysis, patients who underwent the TAVR procedure, were routinely followed up before discharge, and at 30 days and 1 year after the procedure. Clinical adverse events were defined according to the VARC-3 criteria (11).
Statistical Analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences version 25.0.0 (International Business Machines Corporation, Armonk, NY, USA) and GraphPad Prism version 6.0 (GraphPad Software, San Diego, CA, USA). Continuous variables are presented as mean ± SD or median [interquartile range (IQR)] and were analyzed by Student's t-test or Mann-Whitney U-test. Categorical variables are presented as count (percentage). And Pearson's chi-squared test or Fisher exact test were used to analyze the categorical variables. Multivariate logistical regression was used to identify predictors of the HALT, which included co-variables with the p < 0.10 in the univariable logistical regression. Statistical significance was defined at the p < 0.05 with two-tailed tests.
Results
Patients Characteristics
A total of 420 patients underwent the TAVR procedure between August 2015 and March 2020. Among them, 9 patients were excluded because of lacking pre-procedure CT or having a quadricuspid valve, and 29 patients were excluded because of contradictions to contrast agents, death, or loss to follow-up (Figure 1). A total of 371 patients had CT within 30 days post TAVR procedure, of which 20 CT scans were inconclusive because of poor imaging quality. Three hundred and twenty-five patients received CT at 1-year follow-up but 10 CT scans were inconclusive. Finally, 160 patients with BAV involvement and 191 patients with TAV involvement were included for the first CT (within 30 days post the procedure) images analysis. A total of 149 BAVs and 166 TAVs were included for the second CT (at 1-year follow-up; BAV vs. TAV, 12.3 ± 1.1 vs. 12.6 ± 1.9 months) images analysis. A total of 137 BAVs and 159 TAVs had completed CT scans within 30 days and 1-year follow-up.
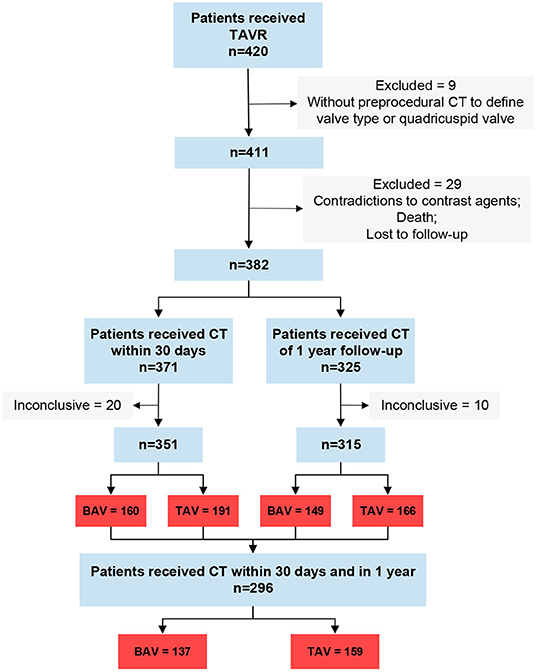
Figure 1. Study flow. Inconclusive, CT can't be analyzed; BAV, icuspid aortic valve; TAV, tricuspid aortic valve.
Baseline demographic and clinical characteristics are summarized in Table 1. A total of 170 BAV cases and 201 TAV cases were enrolled for this study. Patients in BAV group were younger and had a lower risk than patients in TAV group (age: 75.1 ± 6.6 vs. 76.9 ± 6.7, p = 0.013; STS: 6.0 ± 3.7% vs. 7.1 ± 5.1%, p = 0.010). Syncope occurred more frequent in the BAV than the TAV (11.8 vs. 6.0%, p = 0.048).
TAVR Procedure
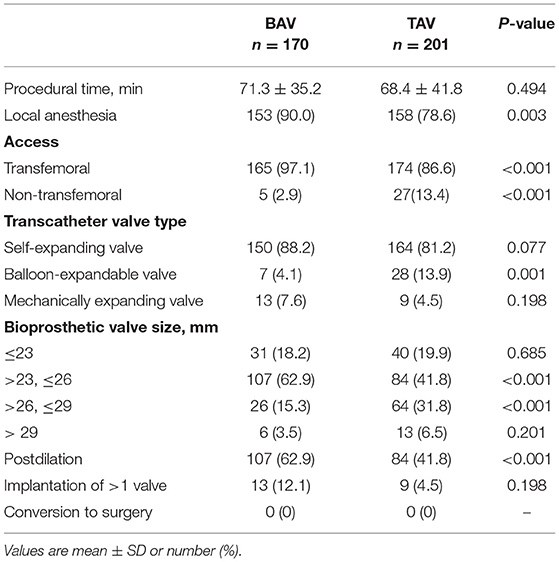
Procedural details were listed in Table 2. There was a higher proportion of local anesthesia (90.0 vs. 78.6%, p = 0.003), transfemoral access (97.1 vs. 86.6%, p < 0.001) and post-dilatation (62.9 vs. 41.8%, p < 0.001) in the BAV group. Many BAV patients were implanted with 23–26 mm valve devices compared with the TAV patients (62.9 vs. 41.8%, p < 0.001). And a higher percentage of 26–29 mm valve devices were implanted in the patients with the TAV (15.3 vs. 31.8%, p < 0.001). No case was converted to surgery of both valves in the BAV and TAV groups.
HALT and RELM
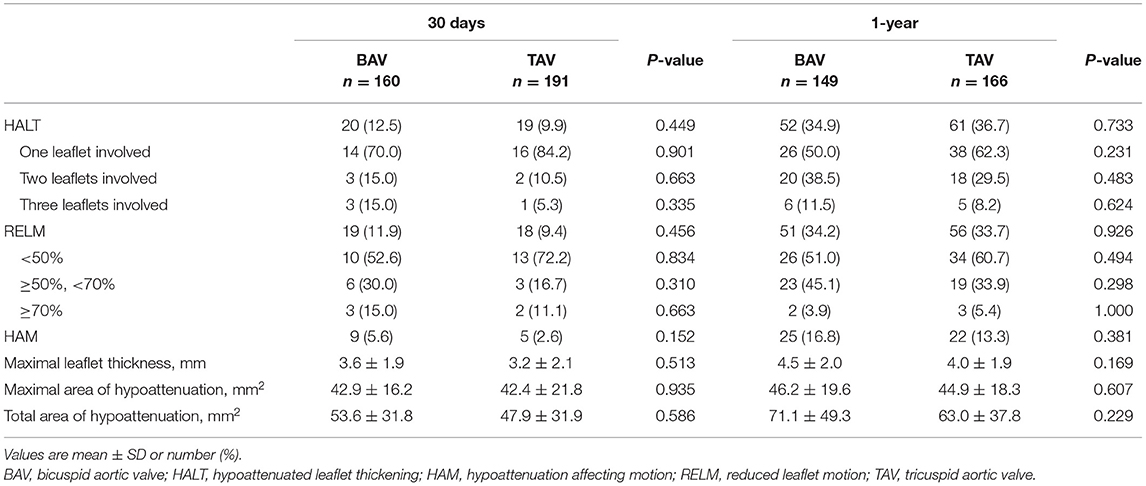
A total of 20 cases in the BAV group and 19 cases in the TAV group had HALT in 30 days (12.5 vs. 9.9%, p = 0.449) (Table 3; Figure 2). Among them, involvement of one leaflet, two leaflets, and three leaflets were 70.0 vs. 84.2% (p = 0.901), 15.0 vs. 10.5% (p = 0.663), and 10.0 vs. 5.3% (p = 0.335) in the BAV and TAV groups, respectively. The occurrence of the RELM in BAV and TAV was 11.9 and 9.4% (p = 0.456) in 30 days. The occurrence of the HAM in BAV and TAV was 5.6 and 2.6% (p = 0.152), respectively. Severe RELM was rare in both groups (1.9 vs. 1.0%, p = 0.663). Maximal leaflet thickness, maximal area of hypoattenuation, and total area of hypoattenuation were comparable in two groups (Table 3).
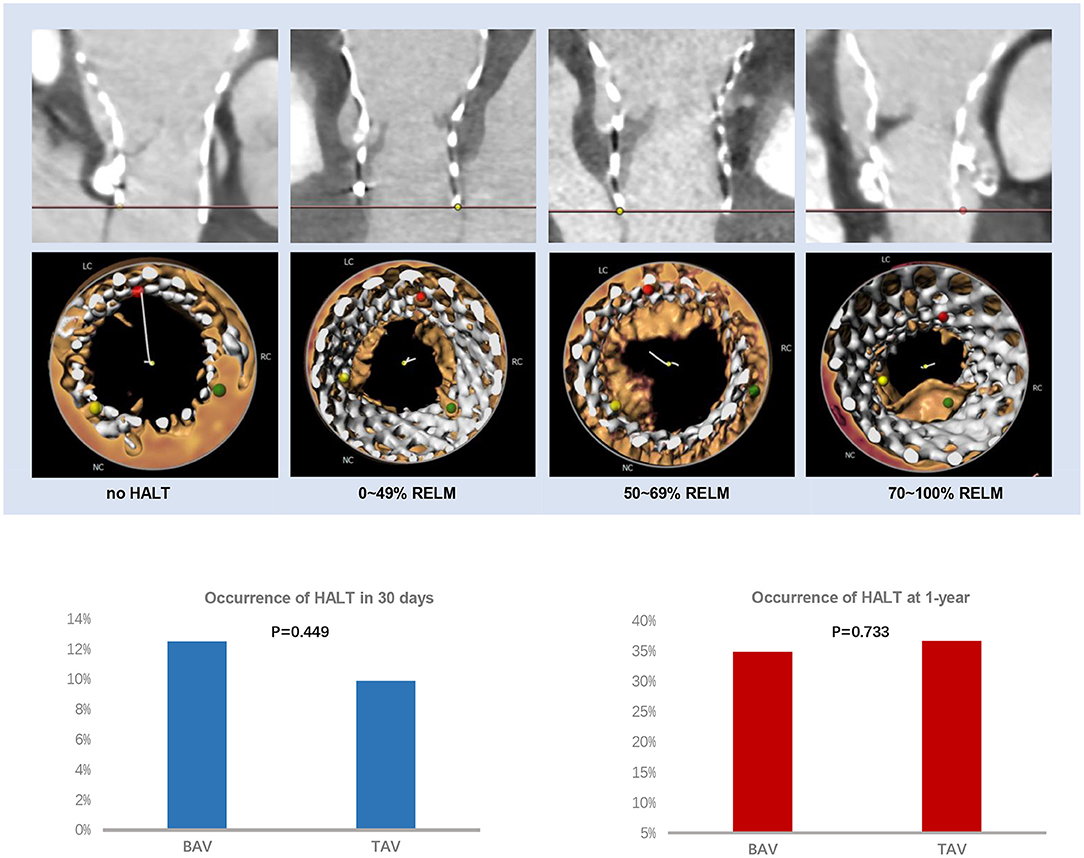
Figure 2. HALT and RELM in BAV and TAV. According to the severity of the leaflet reduced motion, the RELM was graded as mild (<50%), moderate (≥50%, <70%), and severe (≥70%). There was no difference of HALT between the BAV and TAV group within 30 days (12.5 vs. 9.9%, p = 0.449) or at 1-year (34.9 vs. 36.7%, p = 0.733) follow-up. BAV, bicuspid aortic valve; HALT, hypoattenuated leaflet thickening; RELM, reduced leaflet motion; TAV, tricuspid aortic valve.
At 1-year follow-up, there were 52 cases in BAV and 61 cases in TAV with HALT (34.9 vs. 36.7%, p = 0.733), and 51 cases in BAV and 56 cases in TAV with RELM (34.2 vs. 33.7%, p = 0.926) (Table 3; Figure 2). There was no statistical difference with HAM, maximal leaflet thickness, maximal area of hypoattenuation, and total area of hypoattenuation between BAV and TAV.
To eliminate the impact of the device type, we excluded balloon-expandable and mechanically expanding valves. We found the occurrence of HALT was still comparable between the BAV and TAV group within 30 days or at 1 year (BAV vs. TAV, 11.8 vs. 11.6%, p = 0.959; 33.3 vs. 34.8%, p = 0.800) (Supplementary Table 1A). We also compared the occurrence of HALT in the supra-annular bioprostheses (self-expanding valves) and the inter-annular bioprostheses (balloon-expandable and mechanically expanding valves) group (Supplementary Table 1B). The outcomes were still comparable between the self-expanding valves and the balloon-expandable/mechanically expanding valves groups within 30 days or at 1 year (Supplementary Table 1B).
HALT/RELM Evolution
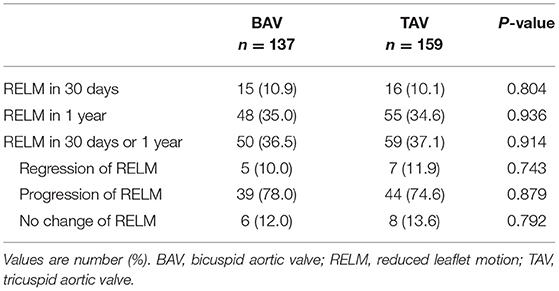
A total of 137 patients with BAV and 159 patients with TAV were evaluated for the evolution of HALT/RELM. Fifty cases in the BAV group and 59 cases in the TAV group (36.5 vs. 37.1%, p = 0.914) had the evolution of HALT/RELM in 30 days or 1 year (Figure 3). Among them, 5 cases in BAV and 7 cases in TAV regressed; 6 cases in BAV and 8 cases in TAV remained stable; most cases in BAV and TAV (78.0 vs. 74.6%, p = 0.879) progressed. Specific data regarding the evolution of HALT/RELM were given in Figure 3; Table 4.
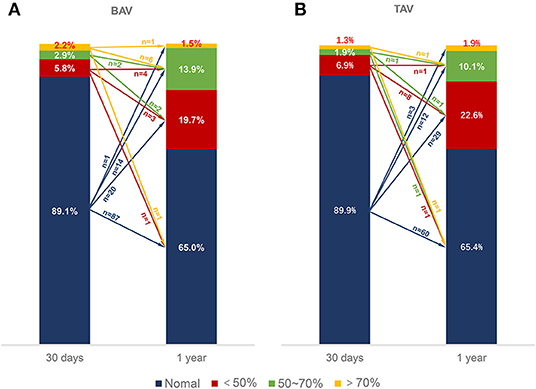
Figure 3. Evolution of RELM. The different color bars represented normal leaflets, <50% RELM, ≥50%, <70% RELM and ≥70% RELM respectively. The different color arrows represented evolution of RELM form 30 days to 1-year follow-up. The numbers upon the color arrows represented the number of patients. BAV, bicuspid aortic valve; RELM, reduced leaflet motion; TAV, tricuspid aortic valve.
Echocardiographic Valve Assessment
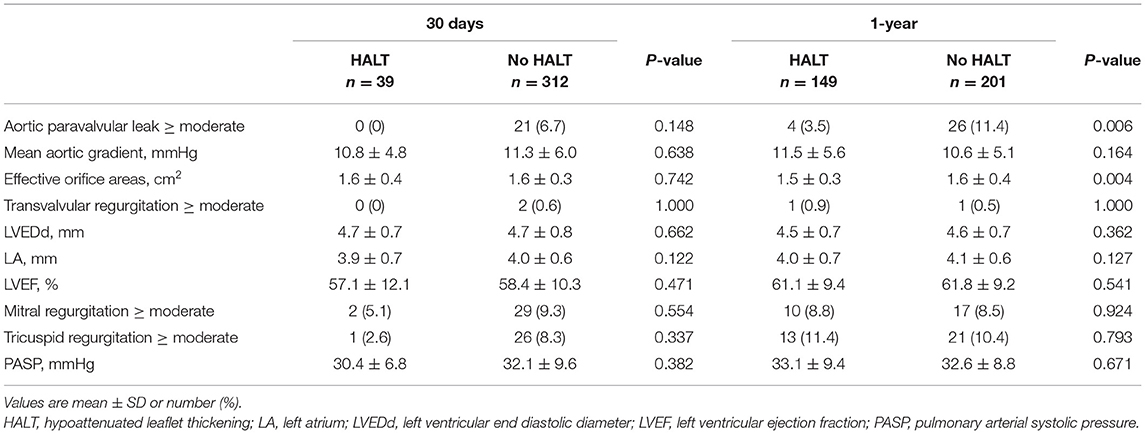
In comparison with the HALT group, the no HALT group had a higher percentage of aortic paravalvular leak of ≥ moderate at 30 days (0 vs. 6.7%) and 1-year (3.5 vs. 11.4%, p = 0.006) follow-up (Table 5). The MAG and EOA values were comparable between the two groups at 30 days (HALT vs. no HALT; 10.8 ± 4.8 vs. 11.3 ± 6.0, p = 0.638; 1.6 ± 0.4 vs. 1.6 ± 0.3, p = 0.724), and still, no difference was observed in the MAG value at 1 year (HALT vs. no HALT; 11.5 ± 5.6 vs. 10.6 ± 5.1, p = 0.164) (Table 5; Figure 4). However, the EOA at 1 year was statistically different between the two groups (HALT vs. no HALT; 1.5 ± 0.3 vs. 1.6 ± 0.4, p = 0.004). Overall, the hemodynamic status was comparable between the HALT and no HALT group at 30 days, but the HALT group had smaller EOA values at 1 year.
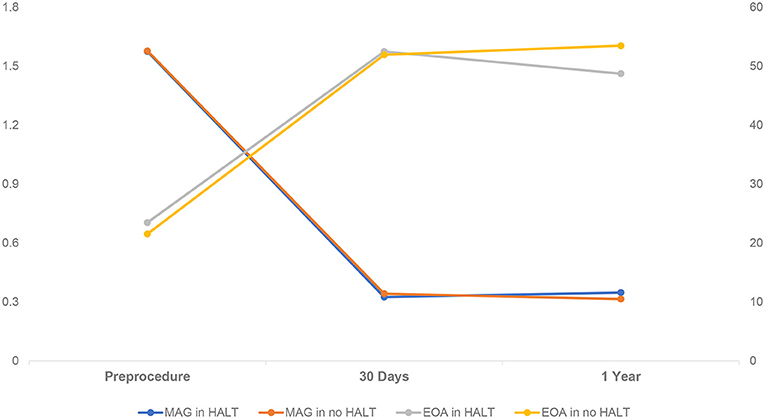
Figure 4. Hemodynamic Change in Patients with HALT or no HALT. There was statistical difference between HALT and no HALT in EOA at 1-year follow-up (1.5 ± 0.3 vs. 1.6 ± 0.4, p = 0.004), but not in MAG (11.5 ± 5.6 vs. 10.6 ± 5.1, p = 0.164). There was no difference between HALT and no HALT in hemodynamic status at 30 days follow-up. EOA, Effective Orifice Areas; HALT, hypoattenuated leaflet thickening; MAG, mean aortic gradient.
Predictors of HALT in BAV and TAV
From the univariate logistical regression, age, body mass index (BMI), Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM), New York Heart Association (NYHA) functional class III/IV, use of anticoagulation, aortic paravalvular leak of ≥ moderate, access, bioprosthetic valve type and D-dimer entered the multivariable logistical regression modeling (Supplementary Table 2). The multivariable logistical regression demonstrated that the anticoagulation and age were independent predictors of both BAV and TAV groups at 1-year (Supplementary Table 3). We didn't find any predictors in the BAV group in 30 days analysis. Transfemoral access and high BMI were protective factors for HALT in the TAV group at 30 days and 1-year, respectively.
Clinical Events
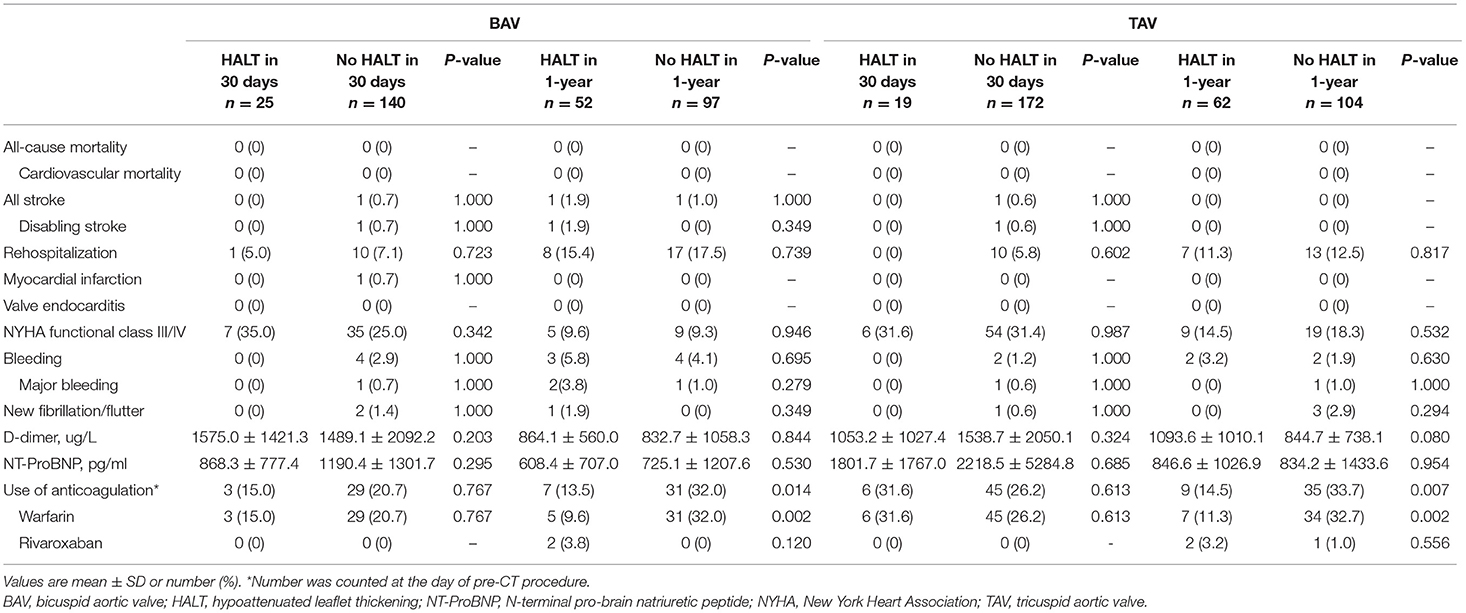
There was no death during the follow-up, including all-cause and cardiovascular mortality in all groups (Table 6). Four cases (3 in BAV and 1 in TAV) had strokes and one case in BAV had a myocardial infarction. Rehospitalization for any reason was comparable in all four groups (Table 5). There was no statistical difference in the NYHA functional class III/IV, bleeding, and new fibrillation/flutter between the HALT and no HALT groups both in the BAV and TAV during the follow-up. In laboratory tests, D-dimer, and N-terminal pro-brain natriuretic peptide (NT-pro-BNP) were not associated with the HALT both in the BAV and TAV. No matter in which group, there was a strong correlation between the HALT and use of anticoagulation at 1-year, but not at 30 days.
Discussion
This study demonstrated that (1) subclinical leaflet thrombosis in BAV and TAV patients was comparable within 30 days or at 1-year; (2) It seemed that the EOA of bioprothesis was different between the HALT and non-HALT group at 1-year follow-up; (3) use of anticoagulation and age were independent predictors both in BAV and TAV; (4) relevant clinical events were similar between the HALT and no HALT groups in BAV and TAV groups.
As the TAVR has been frequently performed in younger and lower-risk patients, the durability of the bioprosthetic valves became a concern in the past years. The SLT was an important cause of bioprosthetic valve dysfunction and compromised the durability of bioprosthetic valves (12). Fortunately, the SLT could be treated and reversed by anticoagulants in many cases (1, 5). Therefore, it may be important to diagnose and treat the SLT to maintain the durability of bioprosthetic valves. At present, some studies have evaluated the leaflet thrombosis of bioprosthetic valves in the TAVR and SAVR procedures, which showed no difference in leaflet thrombosis between the two groups at 1 year (6, 7). Except for durability, Szilveszter et al. found that the SLT was associated with impaired reverse remodeling of left ventricle after the TAVR (13).
Among those younger and lower risk AS patients, the BAV accounted for a large proportion due to the earlier onset in BAV patients. Besides, severe and asymmetric BAV stenosis had some anatomical variations, such as heavily calcified leaflet and the presence of raphe (14), which might have caused under-expansion and malformation of the TAVR stent frame. Those characteristics have increased the concern about leaflet thrombosis and durability in BAV. Waksman et al. found 10.2% HALT from 61 low-risk BAV patients with TAVR at 30 days (8). In this study, the results regarding HALT in BAV patients within 30 days were in line with that previous study. Besides, we explored the differences of HALT between the BAV and TAV groups at early and medium-term follow-up.
In this study, the occurrence of HALT was similar to the outcomes of the prior studies (3, 5, 6). However, we found no difference in HALT between BAV and TAV at early-term (within 30 days) or medium-term (1 year) follow-up. Those anticipated effects of SLT didn't appear to play a role.
In line with the previous studies (15, 16), the MAG value in the HALT or no HALT group was comparable in 1-year follow-up. However, we found that the EOA in the HALT group was smaller than in the no HALT group at 1-year. Of note, MAG in the HALT group was higher than in the no HALT group, although the difference was not significant (HALT vs. no HALT; 11.5 ± 5.6 vs. 10.6 ± 5.1, p = 0.164). As is well-known, there was a high correlation between the EOA and MAG. But the difference of the EOA between the HALT and no HALT group might be enlarged by the square calculation. Therefore, it was reasonable to suppose that the MAG might be significantly higher in the HALT group with a longer follow-up.
Except for the age, the use of anticoagulants was an independent predictor for HALT, regardless of in BAV or TAV. The GALILEO-4D study demonstrated that rivaroxaban reduced the risk of RELM in TAVR patients significantly (5). However, this phenomenon wasn't observed within 30 days in this present study. There might be two reasons: (1) almost all patients who needed anticoagulation received warfarin, which required some time to reach a targeted international normalized ratio (INR); (2) more than 90% of the patients completed first CT scan before discharge so that anticoagulation might not have worked at all. We found the transfemoral access was a protective factor for HALT in the TAV group at 30 days. There was a possible reason involved. Almost patients of non-transfemoral access were received the transapical access, which might affect myocardial contractility due to the surgical trauma during the perioperative period. Low ejection fraction of left ventricle was associated with high occurrence of HALT (2). In this study, we also found that the high BMI was associated with the low occurrence of HALT in the TAV group at 1-year follow-up. We didn't know the nature behind this phenomenon. In previous studies, Abhishek Sharma et al. found patients with higher BMI had better outcomes after TAVR (17). In addition, the aortic paravalvular leak may have been a potential protective factor on SLT (Table 5; Supplementary Table 3), which could have changed the hemodynamic status near the bioprosthesis. This result needs to be confirmed by the studies with the larger sample size.
In previous studies, resolution or regression of the HALT/RELM was observed in half of the patients with HALT from 30 days to 1-year follow-up (6, 7). The rate of resolution or regression of the HALT/RELM was low in the present study. A possible reason was that higher occurrence of HALT/RELM at 30-day follow-up was observed in their studies. However, almost all patients in this study completed the first CT scan before discharge. Lars Sondergaard et al. found regression was more likely to be observed if the first CT scan was obtained at >3months after TAVR (3).
In this study, only a few clinical adverse events were observed. There was no difference between the HALT and no HALT groups in BAV or TAV involvement. Some studies showed a higher rate of stroke, transient ischemic attack (TIA), and thromboembolic complications or stroke, and the TIAs were higher in patients with the HALT than in patients with no HALT (2, 6). However, the relationship between the SLT and clinical adverse events still needs a larger sample size trials to confirm.
There were some limitations in this study. First, this was a retrospective study that couldn't avoid some bias, for example, selective bias. Second, the time point of the CT scan was not the exact timepoint of the SLT occurrence. Third, the sample size was not large enough to assess the differences of clinical adverse events.
Conclusion
The presence of subclinical leaflet thrombosis defined by the CT was comparable between the BAV and TAV in the first year after the TAVR procedure. Age and anticoagulation were the independent predictors of the subclinical leaflet thrombosis at 1 year after the TAVR.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of the Second Affiliated Hospital Zhejiang University School of Medicine. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
GZ: collection, analysis and explanation of data, and drafting of the manuscript. JF: collection, analysis and explanation of data, and revising of the manuscript. DZ, HD, QZ, YH, and YG: collection and analysis of data. XL: conception, design, and revising of the manuscript. JW: conception. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Zhejiang Province Science and Technology Department Key R&D Program (2021C03097).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.790069/full#supplementary-material
References
1. Makkar RR, Fontana G, Jilaihawi H, Chakravarty T, Kofoed KF, De Backer O, et al. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N Engl J Med. (2015) 373:2015–24. doi: 10.1056/NEJMoa1509233
2. Chakravarty T, Søndergaard L, Friedman J, De Backer O, Berman D, Kofoed KF, et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet. (2017) 389:2383–92. doi: 10.1016/S0140-6736(17)30757-2
3. Sondergaard L, De Backer O, Kofoed KF, Jilaihawi H, Fuchs A, Chakravarty T, et al. Natural history of subclinical leaflet thrombosis affecting motion in bioprosthetic aortic valves. Eur Heart J. (2017) 38:2201–7. doi: 10.1093/eurheartj/ehx369
4. Yanagisawa R, Hayashida K, Yamada Y, Tanaka M, Yashima F, Inohara T, et al. Incidence, predictors, and Mid-term outcomes of possible leaflet thrombosis after TAVR. J Am Coll Cardiol Img. (2016) 9:S1936-878X(16)30897-X. doi: 10.1016/j.jcmg.2016.11.005
5. De Backer O, Dangas GD, Jilaihawi H, Leipsic JA, Terkelsen CJ, Makkar R, et al. Reduced leaflet motion after transcatheter aortic-valve replacement. N Engl J Med. (2020) 382:130–9. doi: 10.1056/NEJMoa1911426
6. Makkar RR, Blanke P, Leipsic J, Thourani V, Chakravarty T, Brown D, et al. Subclinical leaflet thrombosis in transcatheter and surgical bioprosthetic valves: PARTNER 3 cardiac computed tomography substudy. J Am Coll Cardiol. (2020) 75:3003–15. doi: 10.1016/j.jacc.2020.04.043
7. Blanke P, Leipsic JA, Popma JJ, Yakubov SJ, Deeb GM, Gada H, et al. Bioprosthetic aortic valve leaflet thickening in the evolut low risk sub-study. J Am Coll Cardiol. (2020) 75:2430–42. doi: 10.1016/j.jacc.2020.03.022
8. Waksman R, Craig PE, Torguson R, Asch FM, Weissman G, Ruiz D, et al. Transcatheter aortic valve replacement in low-risk patients with symptomatic severe bicuspid aortic valve stenosis. JACC Cardiovasc Interv. (2020) 13:1019–27. doi: 10.1016/j.jcin.2020.02.008
9. Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg. (2007) 133:1226–33. doi: 10.1016/j.jtcvs.2007.01.039
10. Jilaihawi H, Asch FM, Manasse E, Ruiz CE, Jelnin V, Kashif M, et al. Systematic CT methodology for the evaluation of subclinical leaflet thrombosis. J Am Coll Cardiol Img. (2017) 10:461–70. doi: 10.1016/j.jcmg.2017.02.005
11. Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, et al. Valve academic research consortium 3: updated endpoint definitions for aortic valve clinical research. J Am Coll Cardiol. (2021) 77:2717–46. doi: 10.1016/j.jacc.2021.02.038
12. Capodanno D, Petronio AS, Prendergast B, Eltchaninoff H, Vahanian A, Modine T, et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2017) 38:3382–90. doi: 10.1093/eurheartj/ehx303
13. Szilveszter B, Oren D, Molnár L, Apor A, Nagy AI, Molnár A, et al. Subclinical leaflet thrombosis is associated with impaired reverse remodelling after transcatheter aortic valve implantation. Eur Heart J Cardiovasc Imaging. (2020) 21:1144–51. doi: 10.1093/ehjci/jez256
14. Yoon SH, Webb JG, Leon MB, Makkar R. Transcatheter aortic valve replacement in bicuspid aortic valve stenosis. Prog Cardiovasc Dis. (2020) 63:482–7. doi: 10.1016/j.pcad.2020.06.007
15. Hein M, Minners J, Jander N, Breitbart P, Stratz C, Pache G, et al. Haemodynamic prosthetic valve performance in patients with early leaflet thrombosis after transcatheter aortic valve implantation. Clin Res Cardiol. (2019) 108:1017–24. doi: 10.1007/s00392-019-01429-7
16. Khan JM, Rogers T, Waksman R, Torguson R, Weissman G, Medvedofsky D, et al. Hemodynamics and subclinical leaflet thrombosis in low-risk patients undergoing transcatheter aortic valve replacement. Circ Cardiovasc Imaging. (2019) 12:e009608. doi: 10.1161/CIRCIMAGING.119.009608
17. Sharma A, Lavie CJ, Elmariah S, Borer JS, Sharma SK, Vemulapalli S, et al. Relationship of body mass index with outcomes after transcatheter aortic valve replacement: results from the national cardiovascular -STS/ACC TVT registry. Mayo Clin Proc. (2020) 95:57–68. doi: 10.1016/j.mayocp.2019.09.027
Keywords: transcatheter aortic valve replacement, subclinical leaflet thrombosis, bicuspid aortic valve, tricuspid aortic valve, hypoattenuated leaflet thickening
Citation: Zhu G, Fan J, Zhou D, Dai H, Zhu Q, He Y, Guo Y, Wang L, Liu X and Wang J (2021) Subclinical Leaflets Thrombosis After Transcatheter Replacement of Bicuspid vs. Tricuspid Aortic Valve. Front. Cardiovasc. Med. 8:790069. doi: 10.3389/fcvm.2021.790069
Received: 06 October 2021; Accepted: 03 December 2021;
Published: 22 December 2021.
Edited by:
Mao Chen, Sichuan University, ChinaReviewed by:
Francesco Sturla, IRCCS Policlinico San Donato, ItalyMara Gavazzoni, University Hospital Zürich, Switzerland
Copyright © 2021 Zhu, Fan, Zhou, Dai, Zhu, He, Guo, Wang, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianbao Liu, liuxb@zju.edu.cn
†These authors have contributed equally to this work
 Gangjie Zhu
Gangjie Zhu Jiaqi Fan
Jiaqi Fan Dao Zhou
Dao Zhou Hanyi Dai1,2
Hanyi Dai1,2  Qifeng Zhu
Qifeng Zhu Yuchao Guo
Yuchao Guo Jian'an Wang
Jian'an Wang