Abstract
We aimed at investigating the distribution and risk of second primary cancers (SPCs) in multiple myeloma (MM) survivors in Germany and Sweden to provide etiological understanding of SPCs and insight into their incidence rates and recording practices. MM patients diagnosed in 1997–2010 at age ≥15 years were selected from the Swedish (nationwide) and 12 German cancer registries. Standardized incidence ratios (SIRs) were used to assess risk of a specific SPC compared to risk of the same first cancer in the corresponding background population. Among 18,735 survivors of first MM in Germany and 7,560 in Sweden, overall 752 and 349 SPCs were recorded, respectively. Significantly elevated SIRs of specific SPCs were observed for acute myeloid leukemia (AML; SIR = 4.9) in Germany and for kidney cancer (2.3), AML (2.3) and nervous system cancer (1.9) in Sweden. Elevated risk for AML was more pronounced in the earlier diagnosis period compared to the later, i.e., 9.7 (4.2–19) for 1997–2003 period versus 3.5 (1.5–6.9) for 2004–2010 in Germany; 3.8 (1.4–8.3) for 1997–2003 versus 2.2 (0.3–7.8) for 2004–2010 in Sweden. We found elevated risk for AML for overall, early diagnosis periods and longer follow-up times in both populations, suggesting possible side effects of treatment for MM patients.
Similar content being viewed by others
Introduction
Overall survival of multiple myeloma (MM) patients has been improved significantly and the improvement in overall survial was very favorable during the last decade among young patients but more recently extends to older patients as well1,2,3,4,5,6,7,8. Consequently, the number of second primary cancers (SPCs) in MM survivors has been steadily increasing1,3,9, For instance, elevated risks of acute myeloid leukemia (AML) and myelodysplastic syndromes in MM survivors are well recorded over last decades1,3,9,10. Although their exact underlying biologic mechanisms have not been well characterized, treatment-related factors may contribute in addition to inherited genetic predisposition and shared non-genetic factors1,3,11,12,13.
Melphalan in combination with prednisone (MP) was the standard treatment for all MM patients since the 1960s for almost thirty years14. However, the outcomes were unsatisfactory with a median survival of one to three years only. The incorporation of high-dose melphalan (HDM) followed by autologous stem cell transplantation, immunomodulatory drugs (IMiDs, such as thalidomide, lenalidomide and pomalidomide) and proteasome inhibitors (PI, e.g., bortezomib and carfilzomib) in MM treatment paved the road towards a sustained disease control and markedly improved survival1,4,15,16,17,18,19,20. Whereas induction therapy incorporating at least one novel agent (IMiD or PI) followed by HDM remains the standard care for younger MM patients (<70 years), MP in combination with either thalidomide or bortezomib, or lenalidomide in combination with dexamethasone, is the preferred treatment for elderly MM patients21,22,23.
Whether or not treatment of MM patients will increase the risk of other SPCs remains under-investigated and, population-based large-scale studies are highly warranted because previous studies were limited by small numbers of MM patients and of SPCs in MM survivors1,3,9,10. However, these studies demonstrated that exposure to alkylating agents such as melphalan/HDM with or without lenalidomide might increase risk of SPC4. To our knowledge, investigations on the risk of specific SPC in MM survivors in two different populations have not been reported. Therefore, using the latest version of the pooled database from 12 population-based German cancer registries24 and the nationwide Swedish Family-Cancer Database (FCD)25, we aimed at investigating the risk of specific SPCs in MM survivors in the two populations, which provides insight into the etiology of SPCs in MM survivors (particularly regarding possible side effects of MM treatment) and into registration practices of SPCs in the two populations.
Results
Distribution of specific SPCs in MM survivors in the two populations
Overall numbers of patients diagnosed with first primary MM during 1997–2010 and aged ≥15 years were 18,735 in Germany and 7,560 in Sweden, accounting for 1.2% and 1.3% of all first primary cancers (except non-melanoma skin cancer), respectively. Among these patients, 752 and 349 SPCs were detected in Germany and Sweden, respectively. The distribution of specific SPCs after MM is presented in Table 1. Overall, frequency ranking order of a specific SPC was quite similar in Germany and Sweden, i.e., the ranking order of the two most frequent SPCs was identical in Germany and Sweden in the sequence of prostate and colorectal cancers, while the ranking order of other SPCs was generally similar, except for stomach cancer [5th (5.5%) in Germany versus 13th (2.3%) in Sweden)] and cancers of the nervous system [13th (1.6%) in Germany versus 9th (4.9%) in Sweden)]. We found similar distributions of characteristics of MM patients in Germany compared to Sweden, e.g., for percentage of men (51.9% in Germany versus 54.2% in Sweden), mean age at diagnosis [69 years (range: 15–102 years) in Germany versus 70 years (range: 24–98 years) in Sweden], and mean follow-up time after first MM until SPCs, death, or end of the study, whichever came first [2.6 years (range: 0–13 years) in both countries].
Risk of a specific SPC in MM survivors in the two populations
The overall SIR and further stratification by the study period are presented in Table 2. We found SIRs to be elevated in Germany for leukemia (SIR = 1.7; 95% CI, 1.2–2.4; out of 14 cancers in total) only and in Sweden for all SPCs combined [1.3 (1.2–1.4)], kidney cancer [2.3 (1.3–3.7)], nervous system cancer [1.9 (1.1–3.1)] and leukemia [1.6 (1.0–2.4)]; elevated SIR for leukemia in both countries was actually due to AML [4.9 (3.2–7.3) in Germany versus 2.3 (1.2–4.0) in Sweden]. Decreased SIRs at levels ranging from 0.4 to 0.9 were found only in Germany for the combined SPCs and six cancers (colorectal, lung, breast, endometrial, prostate and urinary bladder cancers). Further stratification by study period (Table 2) showed similar SIRs in two study periods in Germany and Sweden, except for the combined SPCs [1.2 (1.1–1.4) in 1997–2003 period versus 0.9 (0.8–0.96) in 2004–2010 period) and colorectal cancer [1.3 (0.9–1.8) versus 0.5 (0.4–0.7)] in Germany; although the number of AML cases was small, elevated risk for AML was more pronounced in the earlier diagnosis period compared to the later, i.e., 9.7 (4.2–19) for 1997–2003 versus 3.5 (1.5–6.9) for 2004–2010 in Germany; 3.8 (1.4–8.3) for 1997–2003 versus 2.2 (0.3–7.8) for 2004–2010 in Sweden. Additionally, SIRs for any SPCs were similar between men and women in both countries (data not shown).
We observed a declining trend of SIRs along with increasing age at diagnosis of first primary MM for most of SPCs in Germany, but not in Sweden (Table 3). Elevated SIRs were found in Germany for diagnosis age <65 years for some cancers such as AML [14 (8.0–24)], stomach cancer [2.4 (1.4–3.8)] and kidney cancer [1.9 (1.1–3.0)] and for diagnosis age at 65–74 years for AML [3.1 (1.1–6.8)] only, while in Sweden elevated SIRs were found for diagnosis age <65 years for kidney cancer [4.2 (1.1–11)] only, for diagnosis age at 65–74 years for the combined SPCs [1.4 (1.1–1.7)] and AML [2.9 (1.2–6.0)], and for diagnosis age ≥75 years for the combined SPCs [1.2 (1.0–1.5)] and nervous system cancer [4.0 (1.5–8.6)]. Nevertheless, decreased SIRs at levels ranging from 0.3 to 0.9 were found only in Germany for some cancers by the stratification of age at diagnosis (Table 3).
The SIRs stratified by follow-up time after first MM (<1 year, 1–4 and ≥5 years) are presented in Table 4. Although there was no specific pattern of SIRs by follow-up time in Germany and Sweden, elevated SIRs were found in Germany for AML in ≥5 years follow-up (8.2) and 1–4 years follow-up (5.0), while in Sweden elevated SIRs were found in ≥5 years follow-up for the combined SPCs (1.3) only, in 1–4 years follow-up for AML (2.7), non-Hodgkin lymphoma (2.2), melanoma (1.9), liver and gallbladder cancer (1.6), and the combined SPCs (1.4), and in <1 year follow-up for kidney cancer (3.1) and nervous system cancer (3.0). Nevertheless, decreased SIRs at levels ranging from 0.2 to 0.9 were found only in Germany for some cancers by the stratification of follow-up time (Table 4).
Additionally, sensitivity analyses restricted to eight German cancer registries with full follow-up period 1997–2010 did not essentially change our results (Appendix Tables 1, 2, 3, , , , , , ). We therefore reported data based on 12 German cancer registries to ensure a larger sample size.
Discussion
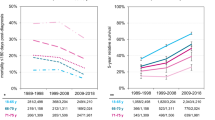
Basic demographic and epidemiological data, such as incidence and survival rates of first primary MM, should be known for the investigation on SPC rates in MM survivors in two populations. Firstly, age-standardized incidence rate of MM (European standard) in 2012 was similar in Germany compared to Sweden for both men and women (5.4 per 100,000 for German men versus 5.3 for Swedish men; 3.5 for German women versus 3.6 for Swedish women)26. Secondly, survival rate after the diagnosis of first MM could be a relevant parameter. Age-standardized 5-year relative survival of MM in the 1995–1999 period was lower in Germany (29%) compared to Sweden (40%)27. Thanks to the widespread introduction of novel agents in addition to conventional chemotherapy and high dose melphalan (HDM)2,19,20, it reached 45% for German men in 201028 versus 47% for Swedish men for the 2009–2012 period and 45% for women from both countries, respectively (NORDCAN)29,30. Thus, MM survival before 2000 was lower in Germany compared to Sweden but the difference has narrowed down thereafter.
A major strength of our study is the design of population-based investigations on risk of a specific SPC in MM survivors in two European populations. Another strength is using large high-quality databases (covering approximately 27 and 9 million people of the German and Swedish population, respectively), including very large samples of MM patients (18,735 from Germany and 7,560 from Sweden). One limitation concerns lack of detailed clinical and/or treatment data, and information on the MM subtypes or prognostic factors such as cytogenetics; however, population-based cancer registries commonly do not have this kind of information. Because sensitivity analyses restricted to eight German cancer registries with full follow-up period 1997–2010 did not essentially change our results (Appendix Tables 1, 2, 3, , , , , , ), we herein presented results according to the data from 12 German cancer registries after taking this difference (four German registries started cancer registration later than 1997) into account for person-year calculations in the German dataset.
Our findings of similar distribution of specific SPCs in the two populations may suggest common etiology for most of SPCs and similarities in the registration for these cancers in the two populations. Our findings of 1.3-fold elevated risk for the combined SPCs in Sweden are consistent with a previous report with partly overlapping data (1986–2005) showing 1.26-fold elevated risk of developing any second malignancies in MM patients, compared to Swedish general population10. Our finding of elevated risk for AML in both countries (4.9 in Germany versus 2.3 in Sweden) is consistent with previous reports1,3,9,10, while elevated risk for AML was also found on longer follow-up and earlier diagnosis periods (Table 4). Exposure to alkylating agents (e.g., melphalan and cyclophosphamide) with or without IMiDs as previously reported might sensibly contribute to both observations1,4. The use of alkylating agents was very common in the earlier diagnosis periods because no other therapeutic options existed. Therefore, these patients might have been exposed to high doses of alkylating agents in the earlier years of diagnosis. However, after the dawn of the new millennium, alkylating agents might not have been used as intensively and frequently as in earlier diagnosis periods because of the introduction of the novel agents. In the later periods, survival from MM diagnosis improved markedly and long-term survivors from both diagnosis periods accumulated. Therefore, AML as long-term side effect from alkylating agents, as described from other cancer entities such as testicular and ovarian cancers31, was more likely to evolve on long-term follow-up. This is supported by the fact that the World Health Organization (WHO) defines subtypes of AML as a second primary malignancy associated with the exposure to alkylating agents32. Nevertheless, non-treatment factors such as inherited genetic predisposition, multiple myeloma-related factors and enviromental factors might also contribute, likely in combination with treatment factors3. Further investigations on AML with larger sample size in MM survivors shall be warranted.
Our findings of elevated SPC risk for kidney and nervous system cancers are principally consistent with previous reports of elevated risk in a single country1,3. Elevated risks for kidney and nervous system cancers were most likely due to increased medical surveillance after the diagnosis of first MM, as suggested by our finding of the elevation found only in <1 year follow up (Table 4). The etiology of SPCs in MM survivors is most likely a multifactorial process and has not been well understood3. While the disease of MM itself is associated with elevated risk of second cancers4,10, other risk factors could also contribute, e.g., characteristics of MM patients, shared lifestyle risk factors between first MM and a SPC, and genetic factors involved in an individual’s susceptibility to development of second primary malignancies, as suggested by other studies3,4,9,30,33.
Decreased SIRs at levels ranging from 0.4 to 0.9 were observed only in Germany for some cancers, including combined SPCs, prostate and colorectal cancers (Table 2), which might be attributed to reporting practices in Germany; prostate and colorectal cancers were two most frequent SPCs in both populations (Table 1). Nevertheless, observed SIRs below 1.0 could result from insufficient medical care rather than incomplete reporting because in the presence of MM with poor prognosis, especially before 2000 in Germany2,27 , no extended diagnostic efforts for SPCs were made in MM patients in Germany. Furthermore, missed deaths data could also contribute to SIR<1.0.
In conclusion, our study provides a comprehensive overview on the risk of a specific SPC in MM survivors, using high-quality data from German and Swedish population-based cancer registries. Although not all SIRs in subgroup-specific analyses reached statistical significance, elevated risks for AML were found for overall, early period and longer follow-up time in both populations, suggesting side effects of treatment for MM patients. Since elevated risks for kidney and nervous system cancers were most likely due to increased medical surveillance after the diagnosis of first MM, there was no consistent evidence on cancer other than AML in MM survivors.
Methods
German data
Details on the pooled German database were described elsewhere24. Briefly, data were originally collected from population-based cancer registries covering 13 of 16 German federal states. According to some criteria closely related to data quality, such as the proportions of cancer cases notified by death certificate only (DCO) or autopsy only (those patients were excluded from the analyses), data from 12 cancer registries, covering a population of 26.7 million people (33% of the total German population), were retained in the pooled German database for further analyses24. According to the rules set up by the International Agency for Research on Cancer (IARC) (16), German cancer registries commonly did not register tumors occurring at the same organ or at the contralateral organ for SPCs and, non-melanoma skin cancers were not continuously collected. For comparability and consistency, second non-melanoma skin cancer and second MM were therefore not included in this study. Cancers were recorded according to the International Classification of Diseases, 10th version (ICD-10)34 and the percentage of microscopically verified cancer diagnosis was larger than 95% in all registries24. Patients who were diagnosed with MM as first primary cancer between 1997–2010, with an age of ≥15 years and with a follow-up information until the end of December 2010 were included in the current analyses.
Swedish data
Swedish FCD was used for the current study; details on this database were described elsewhere25. For comparability and consistency, the same criteria used for German data were adopted for Swedish data, e.g. the definition of primary cancers was recoded and restricted to the study period 1997–2010. Briefly, we used all Swedish MM patients diagnosed 1997–2010, covering approximately 9 million Swedes. DCO cases, second non-melanoma skin cancer and second MM were excluded and, age at diagnosis of first MM and SPC cases was restricted to ≥15 years. Information on cancer cases was retrieved from the Swedish Cancer Registry for the years 1997–2010, relying on separate compulsory notifications from clinicians, pathologists and cytologists35; cancers during the study period were recorded according to both ICD-7 and ICD-10 codes. The Swedish Cancer Registry only records primary malignancies. Metastasized cancers to other sites were only registered at primary sites and, for multiple primary cancers occurring in same organ or same organ system, only clearly separated malignancies were accepted as multiple primaries and registered36. Close to 100% of the registered neoplasms were histologically verified and approximately 98% of second neoplasms were correctly verified according to a re-evaluation study of 209 multiple primary tumors35.
Statistical analyses
For both German and Swedish datasets, standardized incidence ratios (SIRs), calculated as the ratio of observed to expected numbers of cases, were used to assess the risk of a specific SPC in MM survivors. The expected number of a SPC in MM survivors was calculated from the strata-specific first same cancer incidence rates in the Swedish and German general population, respectively, multiplied by the corresponding person-years in MM survivors. Person-years at risk were accumulated for each patient, starting at the date of diagnosis of the first MM (diagnosed from 1997 to 2010), and terminating on the date of a SPC, date of death, date of emigration, or December 31st, 2010 (end of the study), whichever came earliest.
All SIRs for Germany and Sweden were adjusted for three identical variables [sex, age (5-year bands), and calendar period (1995–2000, 2001–2005, and 2006–2010)] and a regional category (12 states in Germany and 4 categories in Sweden). The 95% confidence intervals (CIs) for SIRs were calculated assuming that the cases followed a Poisson distribution. Statistical significance for SIRs to be higher or lower than 1.00 was derived from whether or not the 95% CIs for those SIRs included 1.00. We further stratified patients by years of diagnosis (1997–2003 and 2004–2010), and considered their “restricted” follow-up time in 1997–2003 and 2004–2010, respectively. Additional stratifications by characteristics of cancer patients [sex, age at diagnosis of MM (<65, 65–74 and ≥75 years), and follow-up time after MM (<1 year, 1–4 and ≥5 years)] were also conducted. In order to avoid chance findings, we set up rules for showing results: Firstly, only cancer sites with a total number of a specific SPC ≥5 cases in both countries (except subtypes of leukemia) are presented in the tables. Secondly, although we generally used 1-digital number for reporting SIRs and 95% CIs, for those 95% CIs very close to 1.0, the digital number depends on whether it could be distinguished from 1.0. For instance, 0.8–0.96 will replace 0.8–1.0 in the manuscript text. Additionally, sensitivity analyses restricted to eight German cancer registries with full follow-up period 1997–2010 were conducted because four German registries started cancer registration later than 1997. SAS software (version 9.3, SAS Institute Inc., Cary, NC) was used for the data analyses. Data collection within the German Population-Based Cancer Registries was carried out according to state cancer registry laws and, within this project, only completely anonymous data transferred from the cancer registries were analyzed; although the data in the Swedish Family-Cancer Database were completely anonymous and their use did not entail ethical problems either, ethical approval by the Institutional Review Board, Karolinska Institute was obtained.
Additional Information
How to cite this article: Chen, T. et al. Risk of second primary cancers in multiple myeloma survivors in German and Swedish cancer registries. Sci. Rep. 6, 22084; doi: 10.1038/srep22084 (2016).
References
Landgren, O. & Mailankody, S. Update on second primary malignancies in multiple myeloma: a focused review. Leukemia, doi: 10.1038/leu.2014.22 (2014).
Sant, M. et al. Survival for haematological malignancies in Europe between 1997 and 2008 by region and age: results of EUROCARE-5, a population-based study. The Lancet. Oncology 15, 931–942, doi: 10.1016/S1470-2045(14)70282-7 (2014).
Thomas, A. et al. Second malignancies after multiple myeloma: from 1960s to 2010s. Blood 119, 2731–2737, doi: 10.1182/blood-2011-12-381426 (2012).
Palumbo, A. et al. Second primary malignancies with lenalidomide therapy for newly diagnosed myeloma: a meta-analysis of individual patient data. The Lancet. Oncology 15, 333–342, doi: 10.1016/S1470-2045(13)70609-0 (2014).
Brenner, H., Gondos, A. & Pulte, D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood 111, 2521–2526, doi: 10.1182/blood-2007-08-104984 (2008).
Brenner, H., Gondos, A. & Pulte, D. Expected long-term survival of patients diagnosed with multiple myeloma in 2006–2010. Haematologica 94, 270–275, doi: 10.3324/haematol.13782 (2009).
Pulte, D. et al. Trends in survival of multiple myeloma patients in Germany and the United States in the first decade of the 21st century. British journal of haematology, doi: 10.1111/bjh.13537 (2015).
Pulte, D., Gondos, A. & Brenner, H. Improvement in survival of older adults with multiple myeloma: results of an updated period analysis of SEER data. The oncologist 16, 1600–1603, doi: 10.1634/theoncologist.2011-0229 (2011).
Landgren, O., Thomas, A. & Mailankody, S. Myeloma and second primary cancers. The New England journal of medicine 365, 2241–2242, doi: 10.1056/NEJMc1111010 (2011).
Mailankody, S. et al. Risk of acute myeloid leukemia and myelodysplastic syndromes after multiple myeloma and its precursor disease (MGUS). Blood 118, 4086–4092, doi: 10.1182/blood-2011-05-355743 (2011).
Morgan, G. J. et al. Inherited genetic susceptibility to multiple myeloma. Leukemia 28, 518–524, doi: 10.1038/leu.2013.344 (2014).
Weinhold, N. et al. The CCND1 c.870G>A polymorphism is a risk factor for t(11;14)(q13;q32) multiple myeloma. Nature genetics 45, 522–525, doi: 10.1038/ng.2583 (2013).
Broderick, P. et al. Common variation at 3p22.1 and 7p15.3 influences multiple myeloma risk. Nature genetics 44, 58–61, doi: 10.1038/ng.993 (2012).
Alexanian, R. et al. Combination chemotherapy for multiple myeloma. Cancer 30, 382–389 (1972).
Hideshima, T. et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer research 61, 3071–3076 (2001).
Singhal, S. et al. Antitumor activity of thalidomide in refractory multiple myeloma. The New England journal of medicine 341, 1565–1571, doi: 10.1056/NEJM199911183412102 (1999).
Barlogie, B., Hall, R., Zander, A., Dicke, K. & Alexanian, R. High-dose melphalan with autologous bone marrow transplantation for multiple myeloma. Blood 67, 1298–1301 (1986).
Palumbo, A. & Anderson, K. Multiple myeloma. The New England journal of medicine 364, 1046–1060, doi: 10.1056/NEJMra1011442 (2011).
Armand, J. P. et al. The emerging role of targeted therapy for hematologic malignancies: update on bortezomib and tipifarnib. The oncologist 12, 281–290, doi: 10.1634/theoncologist.12-3-281 (2007).
Moreau, P. et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 24 Suppl 6, vi133–137, doi: 10.1093/annonc/mdt297 (2013).
Palumbo, A. et al. International Myeloma Working Group guidelines for the management of multiple myeloma patients ineligible for standard high-dose chemotherapy with autologous stem cell transplantation. Leukemia 23, 1716–1730, doi: 10.1038/leu.2009.122 (2009).
Benboubker, L. et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. The New England journal of medicine 371, 906–917, doi: 10.1056/NEJMoa1402551 (2014).
Engelhardt, M. et al. European Myeloma Network recommendations on the evaluation and treatment of newly diagnosed patients with multiple myeloma. Haematologica 99, 232–242, doi: 10.3324/haematol.2013.099358 (2014).
Jansen, L. et al. Recent cancer survival in Germany: an analysis of common and less common cancers. International journal of cancer. Journal international du cancer 136, 2649–2658, doi: 10.1002/ijc.29316 (2015).
Chen, T. et al. Effect of a detailed family history of melanoma on risk for other tumors: a cohort study based on the nationwide Swedish Family-Cancer Database. The Journal of investigative dermatology 134, 930–936, doi: 10.1038/jid.2013.460 (2014).
Ferlay, J. et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. European journal of cancer (Oxford, England : 1990) 49, 1374–1403, doi: 10.1016/j.ejca.2012.12.027 (2013).
Sant, M. et al. EUROCARE-4. Survival of cancer patients diagnosed in 1995-1999. Results and commentary. European journal of cancer (Oxford, England : 1990) 45, 931–991, doi: 10.1016/j.ejca.2008.11.018 (2009).
Cancer in Germany 2009/2010. Roboert Kochen Institute, the Association of Population-based Cancer Registries in Germany edn, (Roboert Kochen Institute and the Association of Population-based Cancer Registries in Germany, 2014).
Engholm G., Ferlay J., Christensen N., Kejs A. M. T., Johannesen T. B., Khan S., Leinonen M. K., Milter M. C., Ólafsdóttir E., Petersen T., Trykker H. & Storm H. H. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 7.2 (16.12.2015). Association of the Nordic Cancer Registries. Danish Cancer Society. Available from http://www.ancr.nu, accessed on 23/12/2015.
Nielsen, S. F., Nordestgaard, B. G. & Bojesen, S. E. Associations between first and second primary cancers: a population-based study. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne 184, E57–69, doi: 10.1503/cmaj.110167 (2012).
Bhatia, S. Therapy-related myelodysplasia and acute myeloid leukemia. Seminars in oncology 40, 666–675, doi: 10.1053/j.seminoncol.2013.09.013 (2013).
Vardiman, J. W. et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 114, 937–951, doi: 10.1182/blood-2009-03-209262 (2009).
Winget, M. & Yasui, Y. Variation in risk of second primary cancer. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne 184, 19–20, doi: 10.1503/cmaj.111424 (2012).
Hiripi, E. et al. Survival from common and rare cancers in Germany in the early 21st century. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 23, 472–479, doi: 10.1093/annonc/mdr131 (2012).
Hemminki, K., Li, X., Plna, K., Granstrom, C. & Vaittinen, P. The nation-wide Swedish family-cancer database--updated structure and familial rates. Acta oncologica (Stockholm, Sweden) 40, 772–777 (2001).
Liu, H., Hemminki, K. & Sundquist, J. Renal cell carcinoma as first and second primary cancer: etiological clues from the Swedish Family-Cancer Database. The Journal of urology 185, 2045–2049, doi: 10.1016/j.juro.2011.02.001 (2011).
Acknowledgements
This work was supported by the German Cancer Aid (Deutsche Krebshilfe) [grant number 108257 and 110446]. The study sponsors were not involved in the study design, data collection, data analysis, interpretation of results, writing of the manuscript, and in the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Consortia
Contributions
T.C., H.B. and K.H. designed the research. T.C. performed data analyses. T.C., M.F., H.B., L.J., E.K.M., F.A.C., A.K., K.E., B.H., K.G., A.E., K.S. and K.H. contributed to review of the manuscript. T.C., E.K.M. and K.H. wrote the manuscript. All GEKID Cancer Survival Working Group members (K..G., M.M., A.E., S.L., R.S., S.H., A.N., J.K., E.S., B.H., K.E., H.K., V.M., A.K., N.E., K.K., H.B., L.J. and F.C.) contributed to provide the data and read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Chen, T., Fallah, M., Brenner, H. et al. Risk of Second Primary Cancers in Multiple Myeloma Survivors in German and Swedish Cancer Registries. Sci Rep 6, 22084 (2016). https://doi.org/10.1038/srep22084
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep22084
This article is cited by
-
Effect of autologous hematopoietic stem cell transplant on the development of second primary malignancies in multiple myeloma patients
Blood Cancer Journal (2021)
-
Multiple myeloma: family history and mortality in second primary cancers
Blood Cancer Journal (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.