Abstract
This study reports on plastiglomerate and other new forms of plastic pollution in the tropical marine continent of Indonesia. Twenty-five samples were collected from an island beach in the Java Sea where plastiglomerate, plasticrusts, and pyroplastic were formed by the uncontrolled burning of plastic waste. The most common plastic types were polyethylene and polypropylene (PE/PP), as shown by ATR-FTIR spectroscopy. However, acrylates/polyurethane/varnish (PU) and a copolymer of styrene and acrylonitrile were found as well. This suggests that plastiglomerates can form from a wider variety of plastic polymers than previously reported. FTIR analysis also indicates thermo-oxidative weathering, making the charred plastic more brittle and susceptible to microplastic formation. A subset of the samples was analyzed for associated chemical contaminants. One plastiglomerate with a PU matrix showed high concentrations of phthalates. All samples had high concentrations of polycyclic aromatic hydrocarbons (PAHs), likely due to the burning of the plastic in open fires. The burning leads to a change in the physical and chemical properties of the plastics contained in the plastiglomerates. Plastiglomerate and plastic waste of similar origin are therefore often more weathered and contaminated with organic pollutants than their parent polymers. The highest PAH concentration was found in a plastitar sample. Plastitar is defined as an agglomerate of tar and plastics that adheres to coastal rocks. In contrast, our study documents a more mobile, clastic plastitar type. This clastic plastitar could pose an additional ecological risk because of its mobility. These new types of plastic pollution could be an important vector for chemical contamination of nearby coastal habitats such as coral reefs, seagrass meadows, and mangroves.
Similar content being viewed by others
Introduction
The accumulation of plastic waste has become a global problem since the beginning of mass production of plastic in the 1950s. Thirty-three billion tons of plastic are expected to be produced worldwide by 20501. A significant portion of this ends up in the oceans, primarily due to mismanagement2. Plastic waste is the most important fraction of marine litter and persists in the environment due to its longevity and resistance to weathering and abrasion. The major impacts of plastic debris in the marine environment include entanglement or entrapment and ingestion by marine fauna3,4,5. In addition, marine plastic debris can be a vector for toxic persistent organic pollutants (POPs) via being ingested by invertebrates and transported to higher trophic levels, increasing the potential for bioaccumulation6,7,8.
Recently, new types of plastic formation with potential additional environmental impacts and chemical hazards have been introduced, known as plastiglomerate9. Plastiglomerate was initially described as a mixture of molten plastic with natural and artificial debris, divided into clastic and in situ types9. The definition of plastiglomerate has since been expanded beyond its original classification. Clastic plastiglomerate is described as scattered loose sediment lying on the surface of a beach, often near the vegetation line or buried beneath the sand10. In situ plastiglomerate is formed by molten plastic filling the vesicles and fractures of rocks10. Pyroplastic and plasticrusts are subtypes of plastiglomerate10. Their source is mainly attributed to the informal burning of plastic waste and campfires10,11. Pyroplastics are melted or burned plastics that have lost their original shape and color and sometimes resemble natural rocks11. Pyroplastic is usually formed from plastics without significant additional material, so its bulk density is often low enough to be buoyant in seawater11. Due to their buoyancy and durability, pyroplastics are discussed as a significant vector for alien invasive species12. The term plasticrust was first introduced for pieces of plastic encrusting the surface of rocks13. These plasticrusts were interpreted to form by scouring ropes and other plastic items on rocks in the intertidal zone13,14. Later the term was also used to describe crusts of molten plastic partly covering boulders and larger rocks on a beach15, very similar to the in situ plastiglomerate described by Corcoran et al.9. The agglutination of molten plastic with other natural components, such as rocks, increases their bulk density and limits their ability to be transported by wind or currents. This increases their potential to be buried and preserved, eventually becoming part of the Anthropocene record9. Another new plastic pollution type called plastitar does not conform to the previous classification. Plastitar is formed mainly from plastics and tar but may contain natural and artificial materials16. The observed plastitar was attached and immobilized on the surface of nearshore marine rocks16.
Plastiglomerates were first discovered on the beach of Hawaii9 and later studied in other parts of the world, such as Peru, the Canary Islands, England, Japan and Madeira (northeast Atlantic). Their polymers were mainly identified as polyethylene (PE), polypropylene (PP), and polyethylene terephthalate (PET)9,11,13,15,16,17,18, and they are mainly derived from packaging materials and marine ropes9,13,15,18. Exposure to sunlight and wave action can lead to their disintegration into smaller plastic pieces that may enter the marine food web, with unknown consequences for marine biota11,12,15. Thus, they pose a new challenge to the global plastic pollution problem. It has been postulated that this new plastic formation could be a vector for persistent organic pollutants released by the plastic-burning process9,15,19. On tropical beaches, this would add to the anthropogenic stress on tropical coastal ecosystems such as coral reefs, seagrass meadows, and mangroves 20,21,22,23,24,25, which are already under pressure from other harmful anthropogenic impacts. However, data on organic chemical contaminants in plastiglomerate and other similar forms of plastic pollution are hitherto missing.
In this work, we analyze for the first time the abundance and polymer type of plastiglomerate, pyroplastics, plasticrust, and plastitar from a part of the tropical marine continent of Indonesia. Presence of plastic waste can increase disease susceptibility in reef-building corals26, which may have negative consequences for reef-associated organism and people27. It is shown that this new type of plastic pollution is associated with persistent organic pollutants that could threaten the nearby already degraded coral reef system. A better understanding of this new type of plastic debris specific characteristics will therefore help guiding the worldwide marine conservation policy to protect tropical coastal ecosystems.
Methods
Study area and oceanographic setting
The northern coast of Panjang Island in the Java Sea, Indonesia, was selected to investigate the presence of plastiglomerates and similar types of plastic pollution (Fig. 1). The site was chosen because it is adjacent to the three typical tropical coastal habitats: Coral reefs, seagrass meadows and mangroves. The beach is separated from a fringing reef by a shallow lagoon about 60 m wide with abundant seagrass meadows. The coral reefs in the northern part of Panjang Island are in moderate condition and consist of branching, tabular and massive corals27. Along the beach, the substrate is mainly rubble from branching corals. Towards the east, the beach transitions to a muddier substrate covered by a mangrove ecosystem (Fig. 1). Any plastic pollution present at the beach therefore could potentially contaminate all three tropical coastal ecosystems.
(a) The study area is located in the Java Sea, Indonesia, as indicated by the red arrow. (b) Panjang Island (red square) is located in the north of Banten Bay. Note the turbidity of the water in Banten Bay due to sediment load from the rivers. (c) Sampling site on the northern shore of Panjang Island (red line). Satellite image copyright: ESRI Satellite using QGIS ver.3.16.9.
Panjang Island is located in Banten Bay, a shallow, semi-enclosed body of water generally 2–20 m deep. There are twelve islands in Banten Bay, of which Panjang Island is the largest (8.2 km2) and has more than 4000 inhabitants. Seven rivers flow into Banten Bay and contribute sand and mud to the seabed28. Seagrass, mangrove, and coral reef ecosystems dominate the coastal area. The most extensive seagrass cover is found around Panjang Island29. Mangroves are typical for beaches with muddy substrates28. There are coral reefs on Panjang Island's west, north, and east coasts, but their condition is relatively poor28. The beaches in Banten Bay are composed of coral debris and shell fragments of marine biota28, similar to other beaches in the Java Sea close to coral reef ecosystems30,31.
The monsoon climate influences Banten Bay in the Java Sea. Around Panjang Island, current velocities were measured between 0–2 and 0–2.4 km/h during the east and west monsoons, respectively. These weak ocean currents are responsible for sediment transport in the semi-enclosed Banten Bay28. Salinity in Banten Bay ranges between 29 and 32 ‰ and sea surface temperatures are between 28 and 30 °C. Tides in Banten Bay are predominantly semidiurnal and microtidal, with a mean tidal range of 0.50 m32.
Studies of marine debris in Banten Bay have found that plastic debris is a significant problem33,34. The movement of marine debris depends on the hydrodynamics within the semi-enclosed bay34. Plastic items such as PET bottles, plastic packagings such as cups and lids, and microplastics are common33,34. Model simulations indicate that 41% of the Banten Bay area will be covered with plastic litter by 2028 if no immediate action is taken34.
Sampling
Samples were collected from a wave-exposed beach facing the open sea. The study site is located in a remote coastal area approximately 1 km north of the nearest village and is characterized by limited fishing activity. Three parallel, alongshore transects were surveyed to cover the entire beach from the shoreline to the vegetation line, including the upper intertidal and supratidal zones. Sampling was conducted at high tide. The three alongshore transects have a length of about 500 m and a width of 5 m. Thus, a total area of 2500 m2 was investigated. The entire beach surface was visually examined for plastic debris or abandoned campfire sites. The potential new plastic waste types were identified by visual inspection and comparison with figures and descriptions from published studies and were categorized into plastiglomerate9,10, pyroplastic11,12, plasticrust13,14,15, and plastitar16. The collected samples were subsequently stored in Ziplock bags for further analysis. A total of 32 samples of suspected new plastics were collected.
Basic properties
The samples were photographed using a digital camera, and their maximum length, width, and height were measured with a digital caliper. The weight of each piece was measured using a precision balance (BCE623I-1S, Sartorius Lab Instruments, Göttingen, Germany). Floating tests were performed with artificial seawater (34 ‰) prepared from table salt. Each sample's color, composition, and degree of burning (melted/charred) were recorded. The suspected source of the plastic was identified where possible. Distinguishing the source of the plastic is possible when some parts of the matrix were not fully melted but still retained their original color and shape15. The samples were further classified into four categories: plastiglomerate10, pyroplastic10,11, plasticrust10,13,15, and plastitar16 according to the criteria outlined in previous studies10,11,13,15,16 and stated in the introduction.
Infrared spectroscopy analysis
The dominating polymer type in each sample (n = 25) has been identified using Fourier-transform infrared (FTIR) spectroscopy35. A small plastic chip was cut out of the sample using a scalpel. The plastic polymer was examined via attenuated total reflectance ATR-FTIR spectroscopy (Vertex 80v, Bruker Optics GmbH, Ettlingen, Germany) equipped with a Platinum A225/Q diamond ATR unit (Bruker Optics GmbH, Ettlingen, Germany). Using OPUS 7.5 software (Bruker Optics GmbH, Ettlingen, Germany), the spectra were recorded in the 400–4000 cm−1 wavenumber range by co-adding 32 interferometer scans. The spectral resolution was set to 4 cm−1, and no ATR spectral intensity correction was applied to ensure comparability with the plastic spectra database. Each measurement was performed in triplicate, and the averaged spectrum was used for further analysis. More precisely, the same chip has been re-analyzed three times, with a new background scan performed between each measurement. Good signal-to-noise ratios with typical peak absorbances in the order of 0.1 have been observed, resulting in an excellent reproducibility of the spectra. Open Specy open-source software and its onboard spectral library36,37 were used to further pre-process the spectra and polymer identification. In particular, a thresholding iterative polynomial baseline correction method has been applied with the order of the polynomial chosen to mostly remove the baseline drift and broad features in the spectrum. Spectral regions identified to show no or below-threshold signals are set to zero absorbance. This way, a higher weight was put on narrow-bandwidth vibrational signatures characteristic of the different plastic polymers. Moreover, sample and instrument-specific signal intensity differences between sample and reference spectra have been removed by using absorbance intensities instead of absorbance, i.e., all spectra have been normalized with respect to the absorbance of the highest peak in the spectrum. The spectral assignment was based on Pearson’s r correlation between sample and reference spectra. The reliability of the assignment has been checked by analyzing measured ATR spectra of plastic samples of commercial PE, PP, and PET. For these validation samples, the Pearson correlation coefficients were in the ranges 0.85–0.99. Therefore, only matches with a Pearson correlation coefficient ≥ 0.85 were accepted for the polymer characterization of all samples.
Organic geochemistry
Six samples of plasticrust (n = 3), plastiglomerate (n = 2), and plastitar (n = 1) were further examined for associated chemical contaminants. The outsides of all samples were cleaned by repetitive washing with hot, deionized water and finally with a solvent mixture of n-hexane/isopropanol (9/1: v/v). After drying, an aliquot was crushed and ground in a mortar and extracted by ultrasonication with dichloromethane (DCM), followed by methanol/DCM (9/1: v/v) to utilize the swelling effect of methanol and finally with DCM. Extracts were combined and dewatered over NaSO4 before being taken to dryness. An aliquot of the total extract was redissolved in hexane/DCM (9/1: v/v) and sorbed to silica gel which was applied onto an SPE cartridge for compound class separation into aliphatic and aromatic hydrocarbons and polar components employing an LC-TECH automated SPE system. Separation afforded an 8 ml SPE column (2.8 g Silica 60 mesh, 25–40 μm) using solvents of increasing polarity. Aliphatic hydrocarbons were eluted with n-hexane, aromatic hydrocarbons were eluted using a mixture of n-hexane and DCM (3:2, v/v), and NSO compounds were eluted with DCM:MeOH (1:1, v/v). Aromatic and polar NSO-fractions were spiked with deuterated pyrene and analyzed by GC/MS using an Agilent 5977MS interfaced to an Agilent 7890 gas chromatograph (GC) equipped with a DB-5MS capillary column (Agilent DB5-MS; 30 m length, 0.25 mm inner diameter, 0.25 μm film thickness). The gas chromatograph oven temperature program was: 70 °C (5 min isothermal) to 140 °C at 10 °C/min, then to 325 °C at 3 °C/min (held isothermally for 7 min). The quadrupole mass spectrometer was operated at an ionization energy of 70 eV in scan mode with 1.0 scans per second in the m/z 50–750 mass range. Compounds were identified based on their mass spectra via comparison with authentic standard mixtures and literature data.
Results
Thirty-two samples were collected on the coastal transect. Seven samples were excluded from further analysis because they did not contain plastic. Specifically, six samples were coral fragments partially covered with tar, and one was a coral fragment covered with algae. Thus, 25 samples containing plastic were identified and selected for further analysis (Table 1).
All samples differed in mass and dimensions but were roughly similar in angularity and degree of plastic melting. Coral fragments are the most important natural component in all samples (Fig. 2). The plastiglomerate samples collected (n = 7) were all of the clastic type10, defined as a mixture of natural and artificial material in a molten plastic matrix of various colors. The plastic matrix of several plastiglomerate and plasticrust samples was burned more intensively and partially showed a black charcoal color. Plasticrust (n = 14) was found exclusively in its clastic form. In all samples except one, the melted plastic partially filled the corallites of the coral skeleton. In one sample, a piece of plastic debris was stuck in a coral corallite. Only a few fragments of pyroplastic (n = 3), melted or burned plastic that has lost its original shape and color11, were present. All pyroplastic samples were in the “early stage of formation,” meaning that the plastic had been recently burned and retained some of its color and original shape15. Several samples were found on the backshore within fire pits used for unregulated waste burning.
Different plastic pollution types with variable proportions of plastic. (a) Plastiglomerate formed by coral fragments, wood, and a matrix of melted (blue) and partly charred (black) plastic (PJY1-01). (b) Melted plastic encrusting the surface of a coral fragment and filling its corallites, defined as a clastic plasticrust (PJY1-22). (c) Melted plastic matrix with a single rubble-sized coral fragment and carbonate sand, defined as plastiglomerate (PJY1-08). (d) Pyroplastic of faded pink color and low sphericity (PJY1-03). Plasticrust (b), plastiglomerate (a,c), and pyroplastic (d) represent a continuum with increasing plastic content. Plasticrusts (b) and pyroplastic (d) can be formed by fragmentation of plastiglomerate.
A sample consisting of tar-bonded plastic and natural debris was identified as plastitar (n = 1). The sample resembles a tar ball38 but with the addition of extraneous material. In contrast to the original description16, the plastitar was not attached to a rock outcrop but occurred in a clastic variety. However, only the pyroplastic (n = 3) and not the plastitar sample were buoyant during the flotation test.
Most of the molten plastic retained its original color. The most common color observed was blue (48%), followed by green (36%), pink (4%), red (4%), yellow (4%), and white (4%) (Fig. 3). The source material of the plastic that could be identified in this study includes ropes, a cap/tube, packaging material, coarse fibers, and coarse plastic fabric (Table 1).
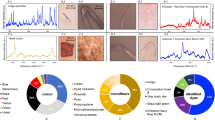
ATR-FTIR spectroscopy (Figs. 4, 5, and 6) confirmed polyethylene (PE, n = 13), polypropylene (PP, n = 9), acrylates/polyurethane/varnish (PU, n = 2; a class of difficult to distinguish polymers as defined by Primpke et al.35 based on a hierarchical cluster analysis of a large dataset of microplastic samples), and a copolymer of styrene and acrylonitrile (SAN, n = 1) as polymers (Fig. 2). The polyethylene was further differentiated into low-density polyethylene (LDPE) and high-density polyethylene (HDPE) based on the presence or absence of a 1377 cm−1 band in the spectra39. In total, 28% of all samples were assigned as HDPE (n = 7) and 24% as LDPE (n = 6). The differentiation between LDPE and HDPE allows a better constraint of the source material since HDPE is mainly used for rigid materials (e.g., cans and pipes), while LDPE is typically used for elastic and transparent products, such as plastic bags and films. Additional absorbance bands, not present in the library spectra of polymers, were commonly observed in the 1000–1200 cm−1, 1700–1800 cm−1, and 3030–3675 cm−1 ranges (Fig. 5). These bands were typically present in the melted plastic. Still, they showed a higher intensity in the charred parts of the same samples (Fig. 5).
Examples of plastiglomerates with an unusual plastic matrix. (a) The FTIR spectrum of the plastic matrix of sample PJY1-08 shows a match of r = 0.98 with a reference spectrum of Acrylates/Polyurethane/Varnish. (b) The FTIR spectrum of the molten plastic matrix of sample PJY1-12 shows an agreement of r = 0.87 with an Acrylate/Polyurethane/Varnish reference spectrum. (c) The FTIR spectrum of sample PJY1-15 confirms the presence of styrene-acrylonitrile copolymer with an agreement of r = 0.98.
Effects of oxidative weathering on the FTIR-Spectrum of an HDPE sample (PJY1-01). Compared to the reference spectrum, the molten (blue) and charred parts of the matrix show additional absorbance bands in the 1000–1200 cm−1, 1700–1800 cm−1, and 3030–3675 cm−1 ranges. These can be attributed to hydroxyl groups, carbonyl groups, and C–O stretching resulting from oxidation. The carbonyl-associated band is only present in the charred part of this sample. The hydroxyl group and C–O stretching bands show higher intensities in the charred region compared to the molten but uncharred part of the sample.
The molten plastic matrix (PU) of a plastiglomerate sample (PJY1-12, Fig. 4) yielded exceptionally high amounts of phthalates in its uncharred portion, which were reduced to less than one-tenth in the charred part of the sample (Table 2). The high phthalate concentration in the uncharred part was accompanied by a high degree of solvent extractability (58% wt). Charring of the plastiglomerate sample reduced the solvent extractability to about 8% [wt] (Table 2). The PAH concentration is significantly increased in this sample’s black, charred portion compared to its green, melted part (Table 2, Fig. 4, sample PJY1-12). In contrast, plastiglomerate samples with a matrix of polyolefin (PE/PP, Fig. 2: PJY1-01, and PJY1-14) show no systematic increase in PAH concentrations in their charred portion (Table 2). The GC/MS analysis indicated that sample PJY1-21 (Fig. 6) is agglutinated by a tar matrix, and was therefore categorized as a plastitar. This sample also showed the highest PAH concentration (Fig. 2).
Discussion
Classification and origin of the new types of plastic pollution
Several new terms were introduced to classify the types of plastic pollution studied9,10,11,12,13,16,18. However, the distinction between plastiglomerate and its variants is sometimes subtle or imprecise. For example, round, molten plastic debris can be referred to as pyroplastic, while similar but more angular plastic with more extraneous materials may be better described as plastiglomerate11. A link between plastiglomerate and pyroplastic was observed on a pebble beach, indicating that pyroplastic can form when a plastiglomerate has lost its clasts40. Similarly, we observed that larger plastiglomerates on the coral-rubble-dominated beach in the Java Sea could break down into smaller fragments due to weathering or wave action, forming plasticrusts, smaller plastiglomerate, and pyroplastic (Fig. 2). These terms, therefore, describe a continuous spectrum of plastic pollution types with an increasing proportion of plastic matrix (Fig. 2). For example, the term plastiglomerate might apply if a single rubble-sized coral fragment and a few sand grains are attached to a relatively large piece of molten plastic (Fig. 2C). However, the term clastic plasticrust might be more appropriate, if the molten plastic forms only a relatively thin veneer on a similar coral clast or fills its corallites.
The original form of five samples of melted plastic debris (24%) was well enough preserved to identify their parent material (Table 1). Four pieces were melted ropes, fibers, or fabric, and one was packaging material. In addition, the plastitar sample contained a plastic cap. The original color of the plastic was at least partially preserved in all samples. The relatively well-preserved shapes and colors indicate that the samples are in their “early and middle stages of formation”15. This is consistent with the fact that several pieces, including pyroplastic, were recovered from fire pits on the backshore area of the beach (see Supplementary 2). These findings imply that the plastics were recently burned in situ and not transported from elsewhere. This contrasts with previous reports11,15, in which pyroplastic samples had a more natural rock-like shape, neutral color, and smooth surface, and were typically found in the intertidal zone.
Therefore, except for the plastitar sample, the new types of plastic pollution described in this study are interpreted to result from unregulated, on-site burning of waste. In fact, the studied coastline in the Java Sea has an order of magnitude higher concentrations of new types of plastic pollution (1 × 10−2 pieces per m2) compared to other sites, e.g., Peru (1 × 10−3–9.74 × 10−5 pieces per m2)15 and Portugal (4 × 10−3 pieces per m2)40. This suggests that even remote beaches of the maritime continent of Indonesia can be highly polluted by plastiglomerate and its variants.
New polymer types in plastiglomerate
ATR-FTIR spectroscopy confirmed that most samples were composed of PE (52%) and PP (36%, Fig. 3). PE and PP are the most common polymers in plastic consumer goods, such as plastic packaging, fishing lines, containers, etc. This is consistent with the observations that the identifiable plastic items such as ropes, fibers, fabric, and packaging materials were either PE or PP (Table 1).
Previous studies on plastiglomerate and its variants have mentioned PE, PP, and PET as polymers11,13,15,17,18,40. This study reports acrylates/polyurethane/varnish and a styrene-acrylonitrile copolymer as additional polymer type in plastiglomerates (Table 1, Fig. 4). The IR-spectrum of sample PJY1-08 (Fig. 4a) yields the best match to the reference spectrum of polymeric n-butyl methacrylate (r = 0.98) and the IR-spectrum of sample PJY1-12 (Fig. 4b) to polyester-based alkylated varnish (r = 0.87), both belonging to the class of acrylates/polyurethane/varnish as defined by Primpke et al35. The IR-spectrum of sample PJY1-15 (Fig. 4c) can be assigned to styrene acrylonitrile (r = 0.98), belonging to the class of polystyrenes. Acrylates are a common ingredient in boat paints because they are known to improve water resistance41. Chipped boat paint is commonly found on beaches and is considered one of the most significant contributors to microplastic pollution in marine habitats worldwide20,42,43,44. Styrene-acrylonitrile copolymer (SAN) is a less commonly used plastic polymer, a tough material known for its heat resistance. The study shows that plastiglomerates and their variants can comprise more polymers than previously known.
Weathering of plastiglomerate and its variants
The unregulated burning of plastic debris in open fire pits caused very variable degrees of melting and charring, even within a single plastic item (e.g., sample PJY1-01, Fig. 2). This was used to assess the effect of burning on plastic weathering. The FTIR spectrum of the blue molten plastic matrix of sample PJY1-01, which in some parts is still recognizable as a maritime rope, is very similar to the polyethylene reference data (Fig. 5, r = 0.98). In addition, the molten plastic shows broad bands in the spectral range between 3200–3600 cm−1 and 1000–1200 cm−1. Broad absorption bands in these regions are associated with hydroxyl groups (3030–3675 cm−1) and are commonly observed in weathered PE45,46,47. An increase in absorption in the 1000–1200 cm−1 range was previously related to the stretching vibration of C–O, also likely as an effect of oxidative weathering45.
It was previously reported that the charring of plastic might lead to a complete obliteration of the polymer FTIR spectrum15,19. In contrast, the samples from Panjang show the typical FTIR spectra of polymers even in the black, charred parts of the samples (e.g., Fig. 5). However, the intensities of the two bands at 3200–3600 cm−1 and 1000–1200 cm−1 are higher in the charred part than in the blue part of the sample, indicating more intense oxidation. The FTIR spectrum of the charred, black matrix exhibits an additional peak at 1717 cm−1, which was not present in the blue, molten part of the sample (Fig. 5). Peaks in the 1700–1800 cm−1 region typically indicate the presence of oxidation products corresponding to the vibration of carbonyl groups, hence may suggest that the polymer has been oxidized48,49. The carbonyl index, which measures the growth in the carbonyl (C=O) absorption relative to a stable reference absorbance band, is commonly used to assess the aging behavior of polymers46,47,50,51. Thus, this peak’s presence confirms that the charred is more oxidized and weathered than the only melted portion of the sample, which has retained its original color. In nature, this weathering is typically attributed to the effect of solar photo-oxidation, i.e., the degradation of polymer chains due to the combined effect of UV light and oxygen47. UV radiation causes the scission of C–H bonds in the polymers and the production of free radicals resulting in the formation of oxidative-functinoal groups such as carboxylic acid and hydroxyl47. However, photo-oxidation is generally limited to the surface layer (100 µm) of polymers47. In contrast, we observed the same spectral features indicative of oxidation in subsamples taken from the interior of the molten plastic matrix. This suggests that the effects of oxidation extend well below this surface layer and, therefore, cannot be solely explained by solar photodegradation. The alternative, thermal degradation of plastic, is often considered unlikely in the environments due to the high temperatures required to start thermo-oxidative reactions47. However, campfire temperatures would be high enough to explain thermal degradation. In general, the mechanism of thermal degradation is comparable to that of photodegradation in oxidizing environments. However, the higher temperatures result in faster reaction rates and larger amounts of free radicals available for chain scission52.
Consequently, plastiglomerate and its variants show a different weathering pattern than other plastic debris. Thermodegradation affects the entire plastic matrix of plastiglomerates, while solar photo-oxidation is dominant on the surface of other plastic waste in the environment. This directly impacts fragmentation behavior, as charred plastic is generally brittle and friable and has been repeatedly cited as a major source of microplastics11,15,17,19,53. To what degree the mechanical properties of the molten but not charred plastic are already influenced by thermo-oxidation is yet unclear and needs further investigation.
Plastitar and persistent organic pollutants
Anthropogenic pollution in coastal regions is not limited to improper and inadequate disposal of plastic waste. There is also a risk of oil entering the marine environment through dumping or accidental spills. This is especially true in marine areas with heavy traffic of oil tankers, such as the Java Sea. Marine tar residues, weathered petroleum compounds derived from oil spills, are found in varying amounts worldwide38.
Plastitar has only been described once before from the Canary Islands16. It is a compound of tar, plastic, and natural components that adhere to and cover coastal rock outcrops16. We found one sample composed of a plastic cap and natural materials (coral fragments, a shell fragment, leaves, and sand) agglutinated in a tar matrix (Fig. 6). Its composition is therefore very similar to the plastitar described by Domínguez-Hernández et al.16, but it is not adhered to a rock outcrop and thus easier transported in the swash zone of the beach. In analogy to the distinction between “in-situ” and “clastic” plastiglomerate9, our sample could be classified as clastic plastitar. ATR-FTIR confirmed that the plastic fragment in the plastitar was LDPE. A previous study showed that PE and PP are common in plastitar16 due to their ubiquity in the marine environment1.
Concerns have been raised that persistent organic pollutants such as PAHs present in tar may transfer toxic, mutagenic, or hormone-like endocrine disruptors to marine organisms16. Currently, there are very few studies on the toxicity of marine tar residues38. In this study, we found a high PAH content in the tar matrix of the plastitar (PJY1-21, Fig. 6, Table 2). The clastic plastitar could pose a high risk to the marine environment because it is more mobile than the plastitar that is adhered to rock outcrops. It can be transported by waves and currents in the swash zone, even though the clastic plastitar in this study is not buoyant. The combination of toxicity in tar and plastics with unknown ecological consequences is a further major concern16.
The concentration of contaminants associated with plastiglomerate and its variants in this study is very high compared to other sites where charred plastic was investigated, e.g., the Maldives19. The overall high PAH levels in all plastiglomerate/-crust samples could possibly be related to the adsorption of PAHs onto plastic from the tar-influenced coast. However, the highest PAH concentrations of > 5 µg/g were measured in two charred samples (Table 2), one from a plasticrust of PP (PJY1-30) and one from the acrylates/PU/varnish matrix of a plastiglomerate (PJY1-12, Fig. 4). The PAH concentrations in the charred portion of sample PJY1-12 were significantly higher compared to the melted but uncharred portion. It is therefore likely that the burning of plastic in fire pits is associated with an increase in PAHs compared to other forms of plastic debris.
One plastiglomerate sample with an acrylates/PU/varnish matrix (PJY1-12, Fig. 4) yielded exceptionally high amounts of phthalates. Phthalates are used as plasticizers, i.e., chemicals that are added to modify the properties of plastic to make them softer or more durable. Even at low concentrations phthalates can act as endocrine disruptors in marine invertebrates, such as bivalves54. Phthalates were detected in the sediments and waters of mangrove ecosystems and can accumulate in mangrove leaves with possible effects on plant biosynthesis and human health55. Their effect on corals is still understudied, but several studies now show that phthalates are a novel, but ubiquitous contaminants in reef sediments20, coral tissue56,57,58 and even the skeleton of reef corals58. Phthalates and other persistent organic pollutants are also known to negatively impact seagrass ecosystem, e.g. by the disruption of metabolic processes24. Phthalates present in microplastic in coastal environments20, could become bioavailable to marine biota if consumed. As discussed before, charred plastic might be an important source of microplastic. This suggests that the degradation of plastiglomerate and their variants could be an important vector for the transfer of persistent organic pollutants in coral reef, seagrass and mangrove environments.
Conclusion
The remote northern beach of Panjang Island in the Java Sea shows the worldwide highest concentration of plastiglomerates and its variants described so far. The most common plastic pollution type are plasticrust, plastiglomerate, and pyroplastic. These items appear to originate from the uncontrolled burning of plastic debris on-site at the beach. Pyroplastics and many plasticrusts likely originate from the decomposition of larger plastiglomerates and form a continuous spectrum of plastic pollution types with an increasing proportion of partially melted plastic. The dominant natural component in these new types of plastic pollution is coral rubble from the close-by reef system.
Due to their ubiquity in the environment, polyolefins (PP/PE) are the most common polymers in the samples studied here. However, acrylates/polyurethane/varnish and styrene-acrylonitrile, are two types of polymers that are described for the first time as a component of plastiglomerates. The FTIR-spectra of the polyolefins show bands in the 3200–3600 cm−1, 1700–1800 cm−1 and 1000–1200 cm−1 region, which are interpreted to results from thermo-oxidation by burning. This thermo-degradation leads to brittle behaviour of the most affected charred portions of the samples, which is believed to contribute to degradation and increased microplastic formation.
One sample of clastic plastitar, which consisted of plastic debris and natural material in a tar matrix, was found in the study area. Considering the intense plastic pollution and heavy traffic of oil tankers in the Java Sea, plastitar may be more common than previously realized. The clastic plastitar could pose a high risk to the marine environment because it is more mobile than the plastitar that is adhered to rock outcrops.
Most of the tested samples in this study showed high content of toxic persistent organic pollutants. The plastitar sample yielded an exceptionally high PAH concentration, while the plastic matrix of an acrylates/polyurethane/varnish sample showed very high phthalate levels. The same plastiglomerate sample also produced high PAHs, with the charred portion’s PAH concentration significantly increasing compared to the non-charred portion. Therefore, partial burning of plastic is likely generated PAHs.
The three most iconic tropical habitats are coral reefs, seagrass meadows and mangrove forests, which provide important ecosystem services. Our study area is located close to all of these habitat types. Local burning of plastic waste leads to changes in their physical and chemical properties, such as the degree of thermo-oxidative weathering and increased PAH concentrations. This could also negatively impact adjacent ecosystems. Plastiglomerates and their variants can act as a source of microplastics and as an important vector for the transfer of toxic persistent organic pollutants (POPs) to coastal habitats. These new types of plastic pollution must therefore be considered in the environmental management of tropical coastal ecosystems such as coral reefs, seagrass meadows, and mangrove forests.
Data availability
The datasets generated during the current study are available in the Supplement 1.
References
Plastic Europe. Plastics: The Facts 2021. An analysis of European plastics production, demand and waste data. (2021).
Jambeck, J. R. et al. Plastic waste inputs from land into the ocean. Science (80-) 347, 768–771 (2015).
Ryan, P. G., Moore, C. J., van Franeker, J. A. & Moloney, C. L. Monitoring the abundance of plastic debris in the marine environment. Philos. Trans. R. Soc. B Biol. Sci. 364, 1999–2012 (2009).
Cole, M., Lindeque, P., Halsband, C. & Galloway, T. S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 62, 2588–2597 (2011).
Galgani, F., Hanke, G., Werner, S. & De Vrees, L. Marine litter within the European marine strategy framework directive. ICES J. Mar. Sci. 70(6), 1055–1064 (2013).
Teuten, E. L. et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. B Biol. Sci. 364, 2027–2045 (2009).
Zarfl, C. & Matthies, M. Are marine plastic particles transport vectors for organic pollutants to the Arctic?. Mar. Pollut. Bull. 60, 1810–1814 (2010).
do Sul, J. A. I. & Costa, M. F. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 185, 352–364 (2014).
Corcoran, P. L., Moore, C. J. & Jazvac, K. An anthropogenic marker horizon in the future rock record. GSA Today https://doi.org/10.1130/GSAT-G198A.1 (2014).
Corcoran, P. L. & Jazvac, K. The consequence that is plastiglomerate. Nat. Rev. Earth Environ. 1, 6–7 (2020).
Turner, A., Wallerstein, C., Arnold, R. & Webb, D. Marine pollution from pyroplastics. Sci. Total Environ. 694, 133610 (2019).
De-la-Torre, G. E., Dioses-Salinas, D. C., Pizarro-Ortega, C. I. & Santillán, L. New plastic formations in the anthropocene. Sci. Total Environ. 754, 142216 (2021).
Gestoso, I., Cacabelos, E., Ramalhosa, P. & Canning-Clode, J. Plasticrusts: A new potential threat in the Anthropocene’s rocky shores. Sci. Total Environ. 687, 413–415 (2019).
Ehlers, S. M., Ellrich, J. A. & Gestoso, I. Plasticrusts derive from maritime ropes scouring across raspy rocks. Mar. Pollut. Bull. 172, 112841 (2021).
De-la-Torre, G. E. et al. First record of plastiglomerates, pyroplastics, and plasticrusts in South America. Sci. Total Environ. 833, 155179 (2022).
Domínguez-Hernández, C. et al. Plastitar: A new threat for coastal environments. Sci. Total Environ. 839, 156261 (2022).
Ehlers, S. M. & Ellrich, J. A. First record of ‘plasticrusts’ and ‘pyroplastic’ from the Mediterranean Sea. Mar. Pollut. Bull. 151, 110845 (2020).
Santos, F. A. et al. Plastic debris forms: Rock analogues emerging from marine pollution. Mar. Pollut. Bull. 182, 114031 (2022).
Saliu, F. et al. Microplastic and charred microplastic in the Faafu Atoll. Maldives. Mar. Pollut. Bull. 136, 464–471 (2018).
Utami, D. A., Reuning, L., Konechnaya, O. & Schwarzbauer, J. Microplastics as a sedimentary component in reef systems: A case study from the Java Sea. Sedimentology https://doi.org/10.1111/sed.12879 (2021).
John, J., Nandhini, A. R., Velayudhaperumal Chellam, P. & Sillanpää, M. Microplastics in mangroves and coral reef ecosystems: A review. Environ. Chem. Lett. 20, 397–416 (2022).
Pantos, O. Microplastics: Impacts on corals and other reef organisms. Emerg. Top. Life Sci. 6, 81–93 (2022).
Nama, S., Shanmughan, A., Nayak, B. B., Bhushan, S. & Ramteke, K. Impacts of marine debris on coral reef ecosystem: A review for conservation and ecological monitoring of the coral reef ecosystem. Mar. Pollut. Bull. 189, 114755 (2023).
Gerstenbacher, C. M., Finzi, A. C., Rotjan, R. D. & Novak, A. B. A review of microplastic impacts on seagrasses, epiphytes, and associated sediment communities. Environ. Pollut. 303, 119108 (2022).
Walther, B. A. & Bergmann, M. Plastic pollution of four understudied marine ecosystems: A review of mangroves, seagrass meadows, the Arctic Ocean and the deep seafloor. Emerg. Top. Life Sci. 6, 371–387 (2022).
Lamb, J. B. et al. Plastic waste associated with disease on coral reefs. Science (80-) 359, 460–462 (2018).
Worm, B. et al. Impacts of biodiversity loss on ocean ecosystem services. Science (80-) 314, 787–790 (2006).
Rustam, A., Adi, N. S., Mustikasari, E., Kepel, T. L. & Kusumaningtyas, M. A. Karakteristik Sebaran Sedimen dan Laju Sedimentasi Perairan Teluk Banten. J. Segara 14, 137–144 (2018).
Daud, M., Pin, T. G. & Handayani, T. The spatial pattern of seagrass distribution and the correlation with salinity, sea surface temperature, and suspended materials in Banten Bay. IOP Conf. Ser. Earth Environ. Sci. 243, 012013 (2019).
Utami, D. A., Reuning, L. & Cahyarini, S. Y. Satellite- and field-based facies mapping of isolated carbonate platforms from the Kepulauan Seribu Complex. Indonesia. Depos. Rec. 4, 255–273 (2018).
Solihuddin, T., Utami, D. A., Salim, H. L. & Prihantono, J. Sedimentary environment of a modern carbonate platform of Karimunjawa islands. Central Java. Indones. J. Geosci. 6, 57–72 (2019).
DLHK Banten. Laporan Akhir daya Dukung dan Daya Tampung Pulau Panjang Dinas Lingkungan Hidup dan Kehutanan Provinsi Banten. https://dlhk.bantenprov.go.id/upload/article-pdf/Pulau Panjang laporan akhir.pdf (2017).
Falahudin, D. et al. The first occurrence, spatial distribution and characteristics of microplastic particles in sediments from Banten Bay. Indonesia. Sci. Total Environ. 705, 135304 (2020).
Wisha, U. J., Gemilang, W. A., Wijaya, Y. J. & Purwanto, A. D. Model-based estimation of plastic debris accumulation in Banten Bay, Indonesia, using particle tracking: Flow model hydrodynamics approach. Ocean Coast. Manag. 217, 106009 (2022).
Primpke, S., Wirth, M., Lorenz, C. & Gerdts, G. Reference database design for the automated analysis of microplastic samples based on Fourier transform infrared (FTIR) spectroscopy. Anal. Bioanal. Chem. 410, 5131–5141 (2018).
Cowger, W. et al. Microplastic spectral classification needs an open source community: Open specy to the rescue!. Anal. Chem. 93, 7543–7548 (2021).
Cowger, W. & Steinmetz, Z. OpenSpecy: Analyze, Process, Identify, and Share Raman and (FT)IR Spectra. Retrieved Mar 2023 from https://github.com/wincowgerDEV/OpenSpecy-package.
Warnock, A. M., Hagen, S. C. & Passeri, D. L. Marine tar residues: A review. Water Air Soil Pollut. 226, 68 (2015).
Jung, M. R. et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 127, 704–716 (2018).
Ellrich, J. A. & Ehlers, S. M. Field observations in pebble beach habitats link plastiglomerate to pyroplastic via pebble clasts. Mar. Pollut. Bull. 174, 113187 (2022).
Hofland, A. Alkyd resins: From down and out to alive and kicking. Prog. Org. Coat. 73, 274–282 (2012).
Gaylarde, C. C., Neto, J. A. B. & da Fonseca, E. M. Paint fragments as polluting microplastics: A brief review. Mar. Pollut. Bull. 162, 111847 (2021).
Cunningham, E. M. et al. The accumulation of microplastic pollution in a commercially important fishing ground. Sci. Rep. 12, 4217 (2022).
Ehlers, S. M., Ellrich, J. A. & Koop, J. H. E. Microplastic load and polymer type composition in European rocky intertidal snails: Consistency across locations, wave exposure and years. Environ. Pollut. 292, 118280 (2022).
Phan, S., Padilla-Gamiño, J. L. & Luscombe, C. K. The effect of weathering environments on microplastic chemical identification with Raman and IR spectroscopy: Part I. Polyethylene and polypropylene. Polym. Test. 116, 107752 (2022).
Zvekic, M., Richards, L. C., Tong, C. C. & Krogh, E. T. Characterizing photochemical ageing processes of microplastic materials using multivariate analysis of infrared spectra. Environ. Sci. Process. Impacts 24, 52–61 (2022).
Campanale, C., Savino, I., Massarelli, C. & Uricchio, V. F. Fourier transform infrared spectroscopy to assess the degree of alteration of artificially aged and environmentally weathered microplastics. Polymers (Basel) 15, 911 (2023).
Syakti, A. D. et al. Beach macro-litter monitoring and floating microplastic in a coastal area of Indonesia. Mar. Pollut. Bull. 122, 217–225 (2017).
Scott, J. W., Turner, A., Prada, A. F. & Zhao, L. Heterogeneous weathering of polypropylene in the marine environment. Sci. Total Environ. 812, 152308 (2022).
Guadagno, L., Naddeo, C., Vittoria, V., Camino, G. & Cagnani, C. Chemical and morphologial modifications of irradiated linear low density polyethylene (LLDPE). Polym. Degrad. Stab. 72, 175–186 (2001).
ter Halle, A. et al. To what extent are microplastics from the open ocean weathered?. Environ. Pollut. 227, 167–174 (2017).
Hakkarainen, M. & Albertsson, A.-C. Environmental degradation of polyethylene, 177–200 (2004). https://doi.org/10.1007/b13523.
Furukuma, S. A study of ‘new plastic formations’ found in the Seto Inland Sea. Japan. Int. J. Sci. Res. Publ. 11, 185–188 (2021).
Oehlmann, J. et al. A critical analysis of the biological impacts of plasticizers on wildlife. Philos. Trans. R. Soc. B Biol. Sci. 364, 2047–2062 (2009).
da Pontes, A. L. S. et al. Phthalates in Avicennia schaueriana, a mangrove species, in the state biological reserve, Guaratiba, RJ, Brazil. Environ. Adv. 2, 100015 (2020).
Saliu, F., Montano, S., Leoni, B., Lasagni, M. & Galli, P. Microplastics as a threat to coral reef environments: Detection of phthalate esters in neuston and scleractinian corals from the Faafu Atoll. Maldives. Mar. Pollut. Bull. 142, 234–241 (2019).
Montano, S. et al. Spatial variability of phthalates contamination in the reef-building corals Porites lutea, Pocillopora verrucosa and Pavona varians. Mar. Pollut. Bull. 155, 111117 (2020).
Ranjbar Jafarabadi, A., Dashtbozorg, M., Raudonytė-Svirbutavičienė, E. & Riyahi Bakhtiari, A. A potential threat to the coral reef environments: Polybrominated diphenyl ethers and phthalate esters in the corals and their ambient environment (Persian Gulf, Iran). Sci. Total Environ. 775, 145822 (2021).
Acknowledgements
The authors gratefully acknowledge research support from the German Academic Exchange Service (DAAD) (research grant to DAU) and the German Society for Sustainable Energy Sources, Mobility and Carbon Cycles (Georg Hunaeus Award to DAU). Dudi Prayudi and Zulfikar Kartadimadja (BRIN-ITB) are thanked for their support during fieldwork. Birgit Mohr and Angela Trumpf (Kiel) are thanked for photographic and laboratory assistance. We thank the Oceanographic ITB for oceanographic survey equipment. ESRI Satellite, QGIS ver.3.16.9, and Open Specy software were used extensively. This study is a contribution to the Alexander von Humboldt Digital Cooperation Fellowship program of SYC Ref 3.4—IDN/1158893.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Main contributing author: D.A.U. funding acquisition, resources, investigation, formal analysis, visualization, writing—original draft, writing—review and editing. Contribution of co-authors: L.R. resources, conceptualization, investigation, validation, formal analysis, writing—original draft, writing—review and editing. L.S. resources, investigation, validation, formal analysis, writing—review and editing. G.F. resources, formal analysis, validation, writing—review and editing. L.D. investigation, writing—review and editing. A.U.N. investigation, writing—review and editing. A.A.F. investigation, writing—review and editing. S.Y.C. resources, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Utami, D.A., Reuning, L., Schwark, L. et al. Plastiglomerates from uncontrolled burning of plastic waste on Indonesian beaches contain high contents of organic pollutants. Sci Rep 13, 10383 (2023). https://doi.org/10.1038/s41598-023-37594-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-37594-z
This article is cited by
-
A mini-review on plasticrusts: occurrence, current trends, potential threats, and recommendations for coastal sustainability
Environmental Monitoring and Assessment (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.