Abstract
Background
To develop a model for prediction of severe intracranial hemorrhage (ICH) or death based on variables from the first 12 h of age and to compare mortality and morbidities with and without exposure to early indomethacin.
Methods
This retrospective cohort study included extreme preterm (220/7−266/7 weeks) infants born at National Institute of Child Health and Human Development Neonatal Research Network sites. Primary outcome was a composite of severe ICH and/or death.
Results
Of 4624 infants, 1827 received early indomethacin. Lower gestation, lack of antenatal steroids exposure, lower 1-min Apgar, male sex, and receipt of epinephrine were associated with severe ICH or death. Early indomethacin was associated with a lower risk of patent ductus arteriosus, bronchopulmonary dysplasia, and higher risk of spontaneous intestinal perforation.
Conclusions
A model for early prediction of severe ICH/death was developed and validated. Early indomethacin was associated with a lower risk of patent ductus arteriosus and bronchopulmonary dysplasia and a higher risk of spontaneous intestinal perforation.
Clinical trial registration
Not applicable.
Impact
-
Modern data on severe ICH and neonatal morbidities in relation to prophylactic indomethacin are scarce in the published literature.
-
Prophylactic indomethacin was associated with a lower risk of patent ductus arteriosus and bronchopulmonary dysplasia and a higher risk of intestinal perforation.
-
A risk estimator for severe intracranial hemorrhage/death was developed in a large cohort of extremely preterm infants.
-
The risk estimator developed based on a large cohort of patients provides an estimate of severe intracranial bleeding for an individual infant.
Similar content being viewed by others
Introduction
Extremely preterm (EPT) infants are at high risk of mortality and neurodevelopmental impairment (NDI). A recent large study in the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network (NICHD NRN) by Stoll and colleagues (n = 34,636, gestational age [GA], 22–28 weeks) noted an improvement in the survival of infants born at 23–24 weeks’ GA from 2009 through 2012, but survival without major neonatal morbidity did not change significantly.1 The incidence of severe intracranial hemorrhage (ICH) among infants of 23 and 25 weeks’ GA remained unchanged during the study period. Severe ICH is associated with mortality, posthemorrhagic hydrocephalus, and need for ventriculoperitoneal shunt, as well as neurodevelopmental sequelae such as cerebral palsy, and intellectual disability.2,3 Perinatal factors associated with severe ICH among preterm infants include lower GA, male sex, antenatal maternal hemorrhage, lack of antenatal steroid exposure, lower Apgar score, mechanical ventilation, neonatal transport, early-onset sepsis, perinatal acidosis, hypotension, hypercapnia, fluctuations in carbon dioxide concentrations, hypoxemia, pneumothorax, and specific gene mutations.4,5,6,7,8,9,10,11,12
Indomethacin prophylaxis immediately after birth remains controversial despite association with a reduction in the incidence of severe ICH and symptomatic patent ductus arteriosus (PDA) among EPT infants.13,14,15 Despite the potential benefits, early indomethacin has not been consistently associated with long-term beneficial effects on neurodevelopment.14,15,16,17 Two large randomized controlled trials did not note significant adverse effects associated with early indomethacin.13,14 However, more recent studies have noted adverse effects of indomethacin including thrombocytopenia, azotemia, gastrointestinal bleeding, and necrotizing enterocolitis (NEC).18,19 Because of conflicting results for neonatal morbidities and long-term neurologic outcomes, clinicians remain divided into proponents and opponents of early indomethacin for EPT infants.20 We speculated that a targeted use of early indomethacin among infants at high risk of severe ICH/death may be beneficial and that risk estimator for severe ICH using clinical variables available soon after birth may help to target preventive therapies to the most at risk of ICH.
The objectives of the study were (1) develop a model for early (12 h of age) prediction of severe ICH within 28 days or death within 7 days among EPT (220/7–266/7 weeks’ GA) infants, and (2) stratify our cohort on risk of severe ICH or death using our predictive of model and compare risk of ICH and other neonatal morbidities among EPT infants based on exposure to early indomethacin.
Patients and methods
Study design
This was a retrospective study of prospectively collected data from the Generic Database Registry among the participating centers of the NICHD NRN. Total 18 centers were included in the analysis dataset.
Study population
All EPT (220/7–266/7 weeks’ GA) infants who were born at participating centers of the NICHD NRN between July 2012 and August 2017 were eligible. Infants who died within 12 h of birth were excluded. Infants born with major congenital malformations and outborn infants were also excluded.
Maternal data collected included race, marital status, hypertension, acute histologic chorioamnionitis, delivery mode, multiple births, education, and antenatal steroid administration. Neonatal data were collected until death, discharge, or 120 days, whichever occurred first and included birth weight, GA (in completed days), sex, Apgar scores, details of resuscitation in the delivery room, severe ICH21, cystic periventricular leukomalacia, traditional BPD defined as need for supplemental oxygen at 36 weeks’ postmenstrual age, stage IIA or higher NEC based on modified Bell criteria22, and mortality. Severe ICH was defined as intraventricular hemorrhage with ventricular dilation or intraparenchymal hemorrhage (either unilateral or bilateral) noted on head ultrasonography (HUS) performed prior to 28 days after birth.21
Cystic periventricular leukomalacia was defined as the presence of cystic echolucencies in the periventricular white matter by serial HUS. Spontaneous intestinal perforation (SIP) without evidence of NEC was diagnosed on radiography, assessment during surgery, or autopsy.
The primary outcome was a composite of severe ICH within 28 days or death within the first 7 days of age. The combined outcome was used because patients who die within the first 7 days of age are at the highest risk of severe ICH.23,24
Statistical analysis
Continuous variables were described using mean and standard deviation (SD) or median and interquartile range, and categorical variables using frequency and percentage. Perinatal characteristics were compared between infants with and without exposure to early indomethacin; and with and without the primary outcome, using χ2 and Wilcoxon rank-sum tests as appropriate.
For objective 1, infants who received early indomethacin, defined as indomethacin administration within 24 hours of age, were excluded for development and validation of an initial ICH/death prediction model, because early indomethacin has been shown to reduce the incidence of ICH.13,14,15 From all the infants eligible for objective 1, two-thirds of infants from each site were randomly assigned to the model development group and the remaining one-third were assigned to the validation group. The initial prediction model development based on logistic regression included the following variables: birth weight, GA, small for GA status, Apgar score at 1 minute, sex, race, antenatal steroid use, antenatal magnesium sulfate use, maternal antibiotic use, antepartum hemorrhage, pregnancy-induced hypertension, chronic hypertension, maternal health insurance, maternal education, delivery method, time of birth, delivery room intubation, chest compression in the delivery room, delivery room use of epinephrine, admission temperature, surfactant use, and pneumothorax. First, the significant factors were identified using backward elimination method. In step 2, an exhaustive list of variables was considered in the model. The model included statistically significant and/or clinically important variables. The final model selection was based on how a predictor enhanced the predictive ability of the model based on change in the area under the curve. The final model fit was also examined based on the Hosmer–Lameshow test.25
All clinical variables that were used in the final model were available within the first 12 h after birth to predict the primary outcome after 12 h of age. A second model was developed to predict severe ICH among survivors.
For objective 2, using the prediction equation, all infants, with or without early indomethacin, were classified into high- and low-risk groups using a cut-off value selected based on the optimum value of sensitivity and specificity. Mortality and neonatal morbidities were compared in the overall cohort and in high- and low- ICH/death risk categories among infants with and without exposure to early indomethacin, using χ2 and t tests. Generalized linear mixed models were developed to compare effect of early indomethacin in the overall cohort as well as separately in high and low-risk groups, with adjustment for covariates known to affect outcomes including GA, sex, antenatal steroid exposure, and 5-minute Apgar score. Interaction effect between early indomethacin and other predictor variables (risk group and antenatal steroid) were evaluated. Interactions that were not statistically significant were excluded from the final model. Center of birth was included as a random effect. No adjustment was made for multiple comparisons. Two-sided P values were reported throughout, with P < 0.05 considered statistically significant. Statistical analyses were conducted using SAS 9.4 statistical software (SAS Institute, Cary, NC). The Generic Database (GDB) data collection was approved by the institutional review board at each site, with waiver of consent granted at all except 3 sites, which required written or oral parental consent.
Results
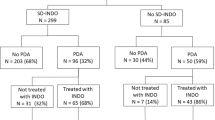
Of the 5835 infants born during the study period, 4624 were included in the analysis (1211 excluded: 305 for congenital anomalies, 191 were outborn, 681 infants died within 12 h, and 34 had missing data). Six hundred eighty-one patients who died within 12 h of age and were excluded from the study cohort had lower GA, mean (SD): 22.9 (1.1) weeks vs. 24.7 (1.1) weeks, lower birth weight, mean (SD): 530.6 (165.1) grams vs. 730.8 (171.2) grams and lower 1-min Apgar score, median (IQR): 1.0 (1–2) vs. 3.0 (1–5), as compared to the infants in the cohort that was included. A higher proportion of patients were born via vaginal delivery; 544 (79.9%) vs. 1666 (36.3%) among the infants who died within 12 hours and were excluded compared to those in the cohort that was included.
Of 4624 infants included, 1827 infants received early indomethacin and 2797 infants did not receive early indomethacin (Fig. 1). The primary outcome of ICH/death occurred in 692/2797 (25%) infants who did not receive early indomethacin, and 458/1827 (25%) infants who did. There were 34 infants with incomplete data for some of the predictors and these infants were excluded from the analysis. Infants who had data available for the primary outcome and infants with missing data were comparable in GA, birth weight, sex, and medical insurance (data not shown).
Infants who received early indomethacin had lower birth weight, GA, 1- and 5-min Apgar scores, and lower rates of maternal antepartum hemorrhage and antenatal exposure to magnesium sulfate. Higher proportion of mothers of infants with early indomethacin was Black and had a college education (Table 1). The use of early indomethacin ranged from 0% in 4 sites to 100% in 1 site, which was reflective of variance in the unit-specific guidelines for the use of early indomethacin among centers.
Maternal and infant characteristics of infants with and without severe ICH/death are described in Table 2. Infants with severe ICH/death had lower GA and birth weight and were more often male, compared to infants without this outcome. For infants with severe ICH/death, rates of exposure to antenatal steroids and antenatal magnesium sulfate were lower and vaginal delivery more frequent, and more infants received delivery room intubation, chest compression, and epinephrine and had lower Apgar scores at 1 and 5 minutes, compared to infants without severe ICH/death. Mean admission temperature was significantly lower in the group with severe ICH/death. Patients who had the primary outcome of severe ICH or death had higher rate of antepartum hemorrhage and pneumothorax as compared to patients without the primary outcome (24.9% vs. 21.1.% and 13.4% vs. 5.9%, respectively). As the timing of pneumothorax was not known, it was not considered in the model. Of 2105 infants without severe ICH/death, 1,666 had no ICH, 242 had grade 1, and 197 infants had grade 2 ICH. Of 692 infants with severe ICH/death: 454 had severe ICH alone, 85 had severe ICH and died within 7 days and 153 died without severe ICH (74 had no ultrasound for ICH or missing data, 58 had no ICH, 9 had grade 1 ICH, and 12 had grade 2 ICH). There were 2 infants who died between 8 and 28 days with the presence of RDS and severe ICH.
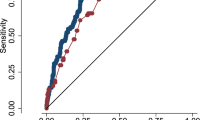
For objective 1, in the initial multivariate logistic regression analysis, lower GA, lack of exposure to antenatal steroids, lower 1-minute Apgar score, male sex, and receipt of epinephrine in the delivery room were associated with severe ICH/death (Fig. 2A). The areas under the receiver operating characteristic curve for the training and validation models were 0.71 (95% CI: 0.68, 0.74) and 0.70 (95% CI: 0.67, 0.74), respectively. A predicted risk cut-off value of 0.24 (sensitivity: 66.7, specificity: 64.1) was selected to define the high- and low-risk groups. The model only used information available within first few hours of age. This could be the reason for the mediocre AUC in the current model. The Hosmer-Lemeshow goodness-of-fit test p-value was 0.90. This p value is nowhere close to statistical significance, thus indicating good model fit. Based on the logistic regression model, we developed a prediction equation for estimating the risk of developing neonatal severe ICH within 28 days or dying within the first 7 days of age using 6 clinical variables available soon after birth. The estimator is available as an EXCEL spreadsheet (Table 4 supplemental file).
a Adjusted markers for severe intracranial hemorrhage within 28 days/mortality within 7 days. ANS antenatal steroids, GA gestational age, LCL lower confidence limit, OR odds ratio, UCL upper confidence limit. b Adjusted markers for severe intracranial hemorrhage within 28 days. ANS antenatal steroids, GA gestational age; LCL lower confidence limit, OR odds ratio, UCL upper confidence limit.
Lower GA, lack of exposure to a complete course of antenatal steroids, lower 1-minute Apgar score, male sex, vaginal delivery, and receipt of epinephrine in the delivery room were associated with severe ICH among survivors (Fig. 2b). The areas under the receiver operating characteristic curve for the training and validation models were 0.69 (95% CI: 0.66, 0.72) and 0.69 (95% CI: 0.65, 0.74). Based on the logistic regression model, we developed a prediction equation for estimating the risk of developing neonatal severe ICH within 28 days of age using 6 clinical variables available soon after birth. The estimator is available as an EXCEL spreadsheet (Table 5 supplemental file).
Neonatal outcomes of EPT infants in relation to early indomethacin are presented in Table 3. Unadjusted and model-adjusted results are provided. None of the interaction terms were statistically significant in the adjusted models, thus, they were removed from the final models. There were no differences in adjusted rates of death, any ICH, severe ICH, and NEC in relation to exposure to early indomethacin. The incidence of PDA, PDA/death was lower, and incidence of SIP was higher, among infants exposed to early indomethacin in the overall group, as well as both high- and low-risk groups. The incidence of moderate or severe traditional BPD (oxygen use at 36 weeks’ postmenstrual age) and moderate or severe BPD/death was lower among infants exposed to early indomethacin in the overall group as well as in the low-risk group. The incidence of physiologic BPD and physiologic BPD/death was lower among infants exposed to early indomethacin in the overall group of infants.
Discussion
Using data from a recent cohort of EPT infants delivered at 18 centers who participated in the NICHD NRN GDB Registry, we developed and validated a prediction model for death or severe ICH using easily available perinatal clinical variables. The current multicenter study is novel by including factors available to clinicians soon after birth (few hours of age) among EPT infants. We have also created an easily usable computational tool in EXCEL that can be used to estimate the probability for ICH for an individual patient based on early predictors. In addition, we examined the risk of various morbidities with early indomethacin both in the entire cohort and stratified by risk of severe ICH. The factors found to be associated with the primary outcome of severe ICH or death within 7 days included lower GA, lack of exposure to a complete course of antenatal steroids, lower 1-min Apgar score, male sex, and receipt of epinephrine in the delivery room. The model in the current study provides an objective and validated estimate of the risk for severe ICH or death among EPT infants soon after birth. As the performance of the model was fair, the model should not be used for clinical purpose unless further external validation is performed. If the model is validated, it could be a valuable tool for early prognostication and parental counseling as well as targeted therapies to reduce the risk of severe ICH.
Two other studies have created prediction models for severe ICH.7,26 Singh et al. developed a prediction model for severe ICH among preterm infants (GA, 23–34 weeks, n = 2917) born at 4 centers using the Vermont Oxford Network database.26 Seven clinical variables available within the first 6 hours of age were associated with severe ICH, including GA, sex, birth weight, any antenatal steroid exposure, mode of delivery, Apgar score at 5 minutes and inborn versus outborn status. Luque et al developed a risk prediction model for severe ICH in preterm infants (n = 6538; birth weight, 500–1249 g) born at the NEOCOSUR Network centers between 2001 and 2010.7 GA, mechanical ventilation, antenatal steroid exposure, 1-minute Apgar score, neonate’s sex, and respiratory distress syndrome were associated with severe ICH. The overall area under the curve for the model was 0.79.
While the variables in all these models are similar, in an inborn EPT population, our model offers the advantages of a consideration of GA in days instead of completed weeks, including infants <500 g and/or <23 weeks, and including incomplete vs complete courses of ANS exposure. Consideration of GA in days vs. completed weeks may be advantageous as the outcome of EPT infants may be closer to infants born in the adjacent week than to those born at the other extreme of the same week. A substantial number of preterm infants are born after receiving an incomplete course of antenatal steroids.27 EPT infants born after exposure to an incomplete course of antenatal steroids have significant differences in survival, ICH, and neurodevelopmental outcomes compared to infants born after no steroids as well as a complete course of antenatal steroids.27,28
Due to conflicting results for neonatal morbidities and long-term neurologic outcomes, clinicians remain divided into proponents and opponents of early indomethacin for EPT infants.20 The current study found that early indomethacin showed no reduction in severe ICH in any risk category. However, there was reduction of PDA and reduction in BPD. Two prior randomized controlled trials showed reduction in ICH and PDA with no difference in BPD or NDI with early indomethacin.13,14,29 Looking at more recent studies, a study from the NICHD NRN by Jensen et al. 30 that included preterm infants born at less than 29 weeks of gestation born between 2008 and 2012 showed that later treatment of PDA was less common among 2587 infants who received early indomethacin compared with 5244 who did not (21.0% vs 36.1%, P < 0.001). After adjusting for potential confounders, use of early indomethacin was not significantly associated with odds of BPD (OR, 0.89; 95% CI, 0.72–1.10), death (OR, 0.80; 95% CI, 0.64–1.01), or death or BPD (OR, 0.87; 95% CI, 0.72–1.05). The difference in the results of current and previous studies could be due to limitations of observational study design; change in practice since the 1990s when the 2 trials were conducted, including increased use of antenatal steroids, change in resuscitation practices for EPT infants, increased use of noninvasive respiratory support; changes over time in obstetric practice and inclusion of smaller and lower GA infants in more modern cohorts. Our finding of reduction in BPD with early indomethacin has been seen in a recently published study by Nelin et al. This observational study reported mortality and neonatal morbidities among EPT infants (GA < 27 weeks) in relation to early indomethacin exposure.31 Infants exposed to early indomethacin (n = 530) had a lower relative risk of mortality and combined outcome of BPD or death, 0.52 (95% CI, 0.37–0.73), and 0.91 (95% CI, 0.85–0.98), respectively, compared to infants who did not receive early indomethacin (n = 141). The exposure to early indomethacin also was not associated with any significant effect on severe ICH similar to this study.31 These findings suggest a potential cardiorespiratory beneficial effect of early indomethacin. This could be secondary to reduction in the rate of symptomatic PDA in the early indomethacin group.
A concerning finding of this study was the association of early indomethacin with an increased incidence of SIP (adjusted odds ratio [OR] = 1.78, 95% confidence interval [CI] = 1.34–2.38), and a marginal increase in incidence of SIP/death (adjusted OR = 1.18, 95% CI = 0.98–1.42), with no significant difference in the incidence of NEC and NEC/death. Similar results were noted in a recent, large observational cohort study (n = 4268, GA < 30 weeks) from Canadian neonatal intensive care units which noted an increased risk of SIP among infants exposed to early indomethacin, after controlling for early feeding (adjusted OR = 2.43, 95% CI = 1.41–4.19).32 This conflicts with the two older randomized controlled trials that did not show a difference in rate of SIP with early indomethacin.13,14
Limitations
The observational design of the study only shows associations between antenatal/perinatal variables and risk of severe ICH. The area under the curve for the prediction model was 0.71 (95% CI: 0.68, 0.74). There are many factors that may occur after 12 h of age and be associated with severe ICH (hypercapnia, fluctuations in blood Pco2, hypotension, hypoxemia, pneumothorax, early-onset sepsis, other unknown confounders, and specific gene mutations) and could not be included in this model.6,7,8,9,10,11,12 Despite these limitations, the area under the curve for the early prediction model was 0.71. These findings are hypothesis generating, and due to multiple outcomes analyzed, we acknowledge the possibility of Type I errors.
The strengths of our study include (1) its derivation from a recent multicenter large cohort of EPT infants, (2) use of clinical variables available soon after birth, and (3) use of prespecified definitions for outcomes. After further external validation, clinicians can use this severe ICH estimator for early prognostication and parental counseling as well as for the study of targeted therapies.
Conclusions and relevance
An early predictive model was developed and validated for severe ICH/death in a large cohort of EPT infants. An estimator of the early predicted probability of severe ICH or death within 7 days may facilitate parental counseling and risk stratification for potential interventions. Exposure to early indomethacin was not associated with a lower risk of severe ICH; it was associated with a lower risk of PDA and bronchopulmonary dysplasia and a higher risk of spontaneous intestinal perforation.
References
Stoll, B. J. et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA 314, 1039–1051 (2015).
Sherlock, R. L., Anderson, P. J. & Doyle, L. W. Neurodevelopmental sequelae of intraventricular haemorrhage at 8 years of age in a regional cohort of ELBW/very preterm infants. Early Hum. Dev. 81, 909–916 (2005).
Luu, T. M. et al. Lasting effects of preterm birth and neonatal brain hemorrhage at 12 years of age. Pediatrics 123, 1037–1044 (2009).
Shankaran, S. et al. Maternal race, demography, and health care disparities impact risk for intraventricular hemorrhage in preterm neonates. J. Pediatr. 164, 1005–1011 e1003 (2014).
Ment, L. R. et al. Gene-environment interactions in severe intraventricular hemorrhage of preterm neonates. Pediatr. Res. 75, 241–250 (2014).
Salhab, W. A., Hynan, L. S. & Perlman, J. M. Partial or complete antenatal steroids treatment and neonatal outcome in extremely low birth weight infants < or =1000 g: is there a dose-dependent effect? J. Perinatol. 23, 668–672 (2003).
Luque, M. J. et al. A risk prediction model for severe intraventricular hemorrhage in very low birth weight infants and the effect of prophylactic indomethacin. J. Perinatol. 34, 43–48 (2014).
Ancel, P. Y. et al. Are maternal hypertension and small-for-gestational age risk factors for severe intraventricular hemorrhage and cystic periventricular leukomalacia? Results of the EPIPAGE cohort study. Am. J. Obstet. Gynecol. 193, 178–184 (2005).
Kaiser, J. R., Gauss, C. H., Pont, M. M. & Williams, D. K. Hypercapnia during the first 3 days of life is associated with severe intraventricular hemorrhage in very low birth weight infants. J. Perinatol. 26, 279–285 (2006).
Linder, N. et al. Risk factors for intraventricular hemorrhage in very low birth weight premature infants: a retrospective case-control study. Pediatrics 111, e590–e595 (2003).
Heuchan, A. M., Evans, N., Henderson Smart, D. J. & Simpson, J. M. Perinatal risk factors for major intraventricular haemorrhage in the Australian and New Zealand Neonatal Network, 1995-97. Arch. Dis. Child. Fetal Neonatal Ed. 86, F86–F90 (2002).
Ryckman, K. K., Dagle, J. M., Kelsey, K., Momany, A. M. & Murray, J. C. Replication of genetic associations in the inflammation, complement, and coagulation pathways with intraventricular hemorrhage in LBW preterm neonates. Pediatr. Res. 70, 90–95 (2011).
Ment, L. R. et al. Low-dose indomethacin and prevention of intraventricular hemorrhage: a multicenter randomized trial. Pediatrics 93, 543–550 (1994).
Schmidt, B. et al. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N. Engl. J. Med. 344, 1966–1972 (2001).
Fowlie, P. W., Davis, P. G. & McGuire, W. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst. Rev. 2010, CD000174 (2010).
Ment, L. R. et al. Outcome of children in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics 105, 485–491 (2000).
Ment, L. R. et al. Neurodevelopmental outcome at 36 months’ corrected age of preterm infants in the Multicenter Indomethacin Intraventricular Hemorrhage Prevention Trial. Pediatrics 98, 714–718 (1996). 4 Pt 1.
Little, D. C. et al. Patent ductus arteriosus in micropreemies and full-term infants: the relative merits of surgical ligation versus indomethacin treatment. J. Pediatr. Surg. 38, 492–496 (2003).
El-Mashad, A. E., El-Mahdy, H., El Amrousy, D. & Elgendy, M. Comparative study of the efficacy and safety of paracetamol, ibuprofen, and indomethacin in closure of patent ductus arteriosus in preterm neonates. Eur. J. Pediatr. 176, 233–240 (2017).
Clyman, R. I., Saha, S., Jobe, A. & Oh, W. Indomethacin prophylaxis for preterm infants: the impact of 2 multicentered randomized controlled trials on clinical practice. J. Pediatr. 150, 46–50 e42 (2007).
Papile, L. A., Burstein, J., Burstein, R. & Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J. Pediatr. 92, 529–534 (1978).
Bell, M. J. et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 187, 1–7 (1978).
Kadri, H., Mawla, A. A. & Kazah, J. The incidence, timing, and predisposing factors of germinal matrix and intraventricular hemorrhage (GMH/IVH) in preterm neonates. Childs Nerv. Syst. 22, 1086–1090 (2006).
Ment, L. R. et al. Intraventricular hemorrhage in the preterm neonate: timing and cerebral blood flow changes. J. Pediatr. 104, 419–425 (1984).
Hosmer, D. W. & Lameshow, S. Applied Logistic Regression. (Wiley, New York, 2013).
Singh, R. et al. A predictive model for SIVH risk in preterm infants and targeted indomethacin therapy for prevention. Sci. Rep. 3, 2539 (2013).
Chawla, S. et al. Outcomes of extremely low birth weight infants with varying doses and intervals of antenatal steroid exposure. J. Perinat. Med. 38, 419–423 (2010).
Chawla, S. et al. Association of neurodevelopmental outcomes and neonatal morbidities of extremely premature infants with differential exposure to antenatal steroids. JAMA Pediatr. 170, 1164–1172 (2016).
Schmidt, B. et al. Indomethacin prophylaxis, patent ductus arteriosus, and the risk of bronchopulmonary dysplasia: further analyses from the Trial of Indomethacin Prophylaxis in Preterms (TIPP). J. Pediatr. 148, 730–734 (2006).
Jensen, E. A. et al. Association between use of prophylactic indomethacin and the risk for bronchopulmonary dysplasia in extremely preterm infants. J. Pediatr. 186, 34–40 e32 (2017).
Nelin, T. D. et al. Outcomes following indomethacin prophylaxis in extremely preterm infants in an all-referral NICU. J. Perinatol. 37, 932–937 (2017).
Stavel, M. et al. Effect of prophylactic indomethacin administration and early feeding on spontaneous intestinal perforation in extremely low-birth-weight infants. J. Perinatol. 37, 188–193 (2017).
Alexander, G. R., Himes, J. H., Kaufman, R. B., Mor, J. & Kogan, M. A United States national reference for fetal growth. Obstet Gynecol. 87 163–168 (1996).
Walsh, M. C. et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 114, 1305–1311 (2004).
Funding
This study is supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland, USA.
Author information
Authors and Affiliations
Consortia
Contributions
S.C.: Developed the protocol, created data collection form, reviewed analysis and drafted first version of the manuscript. G.N.: Contributed to the conception and design, reviewed the protocol, contributed to the analysis, and revised the manuscript critically. A.R.L.: Contributed to the conception and design, reviewed the protocol, contributed to the analysis, and revised the manuscript critically. D.C.: Reviewed the protocol, conducted the data analysis, and revised the manuscript critically. E.F.B.: Contributed to the conception and design, reviewed the protocol, contributed to the analysis, and revised the manuscript critically. N.A.; Contributed to the conception and design, reviewed the protocol, contributed to the analysis, and revised the manuscript critically. W.A.C.: Contributed to the conception and design, reviewed the protocol, contributed to the analysis, and revised the manuscript critically. M.G.: Reviewed the protocol, conducted the data analysis, and revised the manuscript critically. A.D.: Reviewed the protocol, supervised the analysis, and revised the manuscript critically. J.L.T.: Contributed to the conception and design, reviewed the protocol, contributed to the analysis, and revised the manuscript critically. H.H.: Contributed to the conception and design, reviewed the protocol, contributed to the analysis, and revised the manuscript critically. S.S.: Contributed to the conception and design, reviewed the protocol, contributed to the analysis and revised the manuscript critically. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
The Generic Database (GDB) data collection was approved by the institutional review board at each site, with waiver of consent granted at all except 3 sites, which required written or oral parental consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of members and their affiliations appears in the Supplementary Information.
Supplementary information
Rights and permissions
About this article
Cite this article
Chawla, S., Natarajan, G., Laptook, A.R. et al. Model for severe intracranial hemorrhage and role of early indomethacin in extreme preterm infants. Pediatr Res 92, 1648–1656 (2022). https://doi.org/10.1038/s41390-022-02012-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02012-z
This article is cited by
-
Prophylactic indomethacin and the risk of serious pulmonary hemorrhages in preterm infants less than 28 weeks’ gestation
Journal of Perinatology (2024)
-
Prophylactic indomethacin, antenatal betamethasone, and the risk of intestinal perforation in infants <28 weeks’ gestation
Journal of Perinatology (2023)
-
A simple scoring system for prediction of IVH in very-low-birth-weight infants
Pediatric Research (2023)