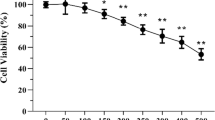
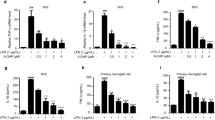
Abstract
Microglia-mediated neuroinflammatory responses are well known to inhibit neurogenesis in the dentate gyrus (DG) of the adult hippocampus, and growing evidence indicates that therapeutic intervention to suppress microglial activation could be an effective strategy for restoring the impaired neurogenesis and memory performance. In the present study, we investigated the effects of water-soluble arginyl–diosgenin analog (Arg-DG) on the adult hippocampal neurogenesis using a central LPS-induced inflammatory mice model, along with the fundamental mechanisms in vivo and in vitro using LPS-stimulated microglial BV2 cells. Arg-DG (0.6 mg/kg) attenuates LPS-impaired neurogenesis by ameliorating the proliferation and differentiation of neural stem cells (NSCs), and prolonging their survival. The impaired neurogenesis in the hippocampal DG triggered the cognitive function, and that treatment of Arg-DG led to the recovery of cognitive decline. Arg-DG also suppressed the production of LPS-induced pro-inflammatory cytokines in hippocampal DG by blocking microglial activation. In in vitro study, Arg-DG inhibited the production of nitric oxide (NO), nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2) expression, and prostaglandin D2 production (PGD2), as well as the pro-inflammatory cytokines, such as interleukin (IL)-6, IL-1β, and tumor necrosis factor alpha (TNF-α). The anti-inflammatory effect of Arg-DG was regulated by NF-κB and MAPK JNK signaling both in vivo, and in LPS-stimulated microglial BV2 cells. Taken together, these results suggest that Arg-DG might have the potential to treat various neurodegenerative disorders resulting from microglia-mediated neuroinflammation.










Similar content being viewed by others
References
Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat Med 4(11):1313–1317. https://doi.org/10.1038/3305
Altman J, Das GD (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124(3):319–335
Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E (2001) Neurogenesis in the adult is involved in the formation of trace memories. Nature 410(6826):372–376. https://doi.org/10.1038/35066584
Bassani TB, Bonato JM, Machado MMF, Coppola-Segovia V, Moura ELR, Zanata SM, Oliveira R, Vital M (2018) Decrease in adult neurogenesis and neuroinflammation are involved in spatial memory impairment in the streptozotocin-induced model of sporadic Alzheimer's disease in rats. Mol Neurobiol 55(5):4280–4296. https://doi.org/10.1007/s12035-017-0645-9
Winner B, Winkler J (2015) Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb Perspect Biol 7(4):a021287. https://doi.org/10.1101/cshperspect.a021287
Singh S, Mishra A, Bharti S, Tiwari V, Singh J, Parul SS (2018) Glycogen synthase kinase-3beta regulates equilibrium between neurogenesis and gliogenesis in rat model of Parkinson's disease: a crosstalk with Wnt and notch signaling. Mol Neurobiol 55(8):6500–6517. https://doi.org/10.1007/s12035-017-0860-4
van Praag H, Kempermann G, Gage FH (1999) Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2(3):266–270. https://doi.org/10.1038/6368
Kempermann G, Kuhn HG, Gage FH (1997) More hippocampal neurons in adult mice living in an enriched environment. Nature 386(6624):493–495. https://doi.org/10.1038/386493a0
Ming GL, Song H (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70(4):687–702. https://doi.org/10.1016/j.neuron.2011.05.001
Carpentier PA, Palmer TD (2009) Immune influence on adult neural stem cell regulation and function. Neuron 64(1):79–92. https://doi.org/10.1016/j.neuron.2009.08.038
Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O (2003) Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A 100(23):13632–13637. https://doi.org/10.1073/pnas.2234031100
Monje ML, Toda H, Palmer TD (2003) Inflammatory blockade restores adult hippocampal neurogenesis. Science (New York, NY) 302(5651):1760–1765. https://doi.org/10.1126/science.1088417
Wu MD, Montgomery SL, Rivera-Escalera F, Olschowka JA, O'Banion MK (2013) Sustained IL-1beta expression impairs adult hippocampal neurogenesis independent of IL-1 signaling in nestin+ neural precursor cells. Brain Behav Immun 32:9–18. https://doi.org/10.1016/j.bbi.2013.03.003
Cacci E, Claasen JH, Kokaia Z (2005) Microglia-derived tumor necrosis factor-alpha exaggerates death of newborn hippocampal progenitor cells in vitro. J Neurosci Res 80(6):789–797. https://doi.org/10.1002/jnr.20531
Vallieres L, Campbell IL, Gage FH, Sawchenko PE (2002) Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci 22(2):486–492
Rosadini CV, Kagan JC (2017) Early innate immune responses to bacterial LPS. Curr Opin Immunol 44:14–19. https://doi.org/10.1016/j.coi.2016.10.005
Rehman SU, Ali T, Alam SI, Ullah R, Zeb A, Lee KW, Rutten BPF, Kim MO (2018) Ferulic acid rescues LPS-induced neurotoxicity via modulation of the TLR4 receptor in the mouse hippocampus. Mol Neurobiol. https://doi.org/10.1007/s12035-018-1280-9
Younger D, Murugan M, Rama Rao KV, Wu LJ, Chandra N (2018) Microglia receptors in animal models of traumatic brain injury. Mol Neurobiol. https://doi.org/10.1007/s12035-018-1428-7
Seong KJ, Lee HG, Kook MS, Ko HM, Jung JY, Kim WJ (2016) Epigallocatechin-3-gallate rescues LPS-impaired adult hippocampal neurogenesis through suppressing the TLR4-NF-kappaB signaling pathway in mice. Korean J Physiol Pharmacol 20(1):41–51. https://doi.org/10.4196/kjpp.2016.20.1.41
Doring C, Regen T, Gertig U, van Rossum D, Winkler A, Saiepour N, Bruck W, Hanisch UK et al (2017) A presumed antagonistic LPS identifies distinct functional organization of TLR4 in mouse microglia. Glia 65(7):1176–1185. https://doi.org/10.1002/glia.23151
Cai B, Seong KJ, Bae SW, Chun C, Kim WJ, Jung JY (2018) A synthetic diosgenin primary amine derivative attenuates LPS-stimulated inflammation via inhibition of NF-kappaB and JNK MAPK signaling in microglial BV2 cells. Int Immunopharmacol 61:204–214. https://doi.org/10.1016/j.intimp.2018.05.021
Kim SJ, Son TG, Park HR, Park M, Kim MS, Kim HS, Chung HY, Mattson MP et al (2008) Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J Biol Chem 283(21):14497–14505. https://doi.org/10.1074/jbc.M708373200
Ajmone-Cat MA, Cacci E, Minghetti L (2008) Non steroidal anti-inflammatory drugs and neurogenesis in the adult mammalian brain. Curr Pharm Des 14(14):1435–1442
Kimura T, Hong Nguyen PT, Ho SA, Tran AH, Ono T, Nishijo H (2009) T-817MA, a neurotrophic agent, ameliorates the deficits in adult neurogenesis and spatial memory in rats infused I.C.V. with amyloid-beta peptide. Br J Pharmacol 157(3):451–463. https://doi.org/10.1111/j.1476-5381.2009.00141.x
Ryu JR, Hong CJ, Kim JY, Kim EK, Sun W, Yu SW (2016) Control of adult neurogenesis by programmed cell death in the mammalian brain. Mol Brain 9:43. https://doi.org/10.1186/s13041-016-0224-4
Tohda C, Urano T, Umezaki M, Nemere I, Kuboyama T (2012) Diosgenin is an exogenous activator of 1,25D(3)-MARRS/Pdia3/ERp57 and improves Alzheimer's disease pathologies in 5XFAD mice. Sci Rep 2:535. https://doi.org/10.1038/srep00535
Zhang X, Xue X, Zhao J, Qian C, Guo Z, Ito Y, Sun W (2016) Diosgenin attenuates the brain injury induced by transient focal cerebral ischemia–reperfusion in rats. Steroids 113:103–112. https://doi.org/10.1016/j.steroids.2016.07.006
Liu W, Zhu M, Yu Z, Yin D, Lu F, Pu Y, Zhao C, He C et al (2017) Therapeutic effects of diosgenin in experimental autoimmune encephalomyelitis. J Neuroimmunol 313:152–160. https://doi.org/10.1016/j.jneuroim.2017.10.018
Xiao L, Guo D, Hu C, Shen W, Shan L, Li C, Liu X, Yang W et al (2012) Diosgenin promotes oligodendrocyte progenitor cell differentiation through estrogen receptor-mediated ERK1/2 activation to accelerate remyelination. Glia 60(7):1037–1052. https://doi.org/10.1002/glia.22333
Lecanu L, Tillement L, Rammouz G, Tillement JP, Greeson J, Papadopoulos V (2009) Caprospinol: moving from a neuroactive steroid to a neurotropic drug. Expert Opin Investig Drugs 18(3):265–276. https://doi.org/10.1517/13543780902762827
Wang S, Wang F, Yang H, Li R, Guo H, Hu L (2017) Diosgenin glucoside provides neuroprotection by regulating microglial M1 polarization. Int Immunopharmacol 50:22–29. https://doi.org/10.1016/j.intimp.2017.06.008
Liao AM, Jung H, Yu JW, Lee DH, Park SS, Cai B, Chun C (2018) Synthesis and biological evaluation of arginyl–diosgenin conjugate as a potential bone tissue engineering agent. Chem Biol Drug Des 91(1):17–28. https://doi.org/10.1111/cbdd.13050
Liu L, Hoang-Gia T, Wu H, Lee MR, Gu L, Wang C, Yun BS, Wang Q et al (2011) Ginsenoside Rb1 improves spatial learning and memory by regulation of cell genesis in the hippocampal subregions of rats. Brain Res 1382:147–154. https://doi.org/10.1016/j.brainres.2011.01.051
Nam SM, Kim JW, Yoo DY, Jung HY, Chung JY, Kim DW, Hwang IK, Yoon YS (2018) Hypothyroidism increases cyclooxygenase-2 levels and pro-inflammatory response and decreases cell proliferation and neuroblast differentiation in the hippocampus. Mol Med Rep 17(4):5782–5788. https://doi.org/10.3892/mmr.2018.8605
Jarlestedt K, Naylor AS, Dean J, Hagberg H, Mallard C (2013) Decreased survival of newborn neurons in the dorsal hippocampus after neonatal LPS exposure in mice. Neuroscience 253:21–28. https://doi.org/10.1016/j.neuroscience.2013.08.040
Mattei D, Djodari-Irani A, Hadar R, Pelz A, de Cossio LF, Goetz T, Matyash M, Kettenmann H et al (2014) Minocycline rescues decrease in neurogenesis, increase in microglia cytokines and deficits in sensorimotor gating in an animal model of schizophrenia. Brain Behav Immun 38:175–184. https://doi.org/10.1016/j.bbi.2014.01.019
Battista D, Ferrari CC, Gage FH, Pitossi FJ (2006) Neurogenic niche modulation by activated microglia: transforming growth factor beta increases neurogenesis in the adult dentate gyrus. Eur J Neurosci 23(1):83–93. https://doi.org/10.1111/j.1460-9568.2005.04539.x
Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J et al (2006) Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci 9(2):268–275. https://doi.org/10.1038/nn1629
Zheng JY, Liang KS, Wang XJ, Zhou XY, Sun J, Zhou SN (2017) Chronic estradiol administration during the early stage of Alzheimer’s disease pathology rescues adult hippocampal neurogenesis and ameliorates cognitive deficits in Abeta1–42 mice. Mol Neurobiol 54(10):7656–7669. https://doi.org/10.1007/s12035-016-0181-z
Farioli-Vecchioli S, Saraulli D, Costanzi M, Pacioni S, Cina I, Aceti M, Micheli L, Bacci A et al (2008) The timing of differentiation of adult hippocampal neurons is crucial for spatial memory. PLoS Biol 6(10):e246. https://doi.org/10.1371/journal.pbio.0060246
Zhang QJ, Li J, Zhang SY (2018) Effects of TRPM7/miR-34a gene silencing on spatial cognitive function and hippocampal neurogenesis in mice with type 1 diabetes mellitus. Mol Neurobiol 55(2):1568–1579. https://doi.org/10.1007/s12035-017-0398-5
Juliandi B, Tanemura K, Igarashi K, Tominaga T, Furukawa Y, Otsuka M, Moriyama N, Ikegami D et al (2015) Reduced adult hippocampal neurogenesis and cognitive impairments following prenatal treatment of the antiepileptic drug valproic acid. Stem Cell Rep 5(6):996–1009. https://doi.org/10.1016/j.stemcr.2015.10.012
Kim YE, Hwang CJ, Lee HP, Kim CS, Son DJ, Ham YW, Hellstrom M, Han SB et al (2017) Inhibitory effect of punicalagin on lipopolysaccharide-induced neuroinflammation, oxidative stress and memory impairment via inhibition of nuclear factor-kappaB. Neuropharmacology 117:21–32. https://doi.org/10.1016/j.neuropharm.2017.01.025
Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D (2015) Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation 12:114. https://doi.org/10.1186/s12974-015-0332-6
Borsini A, Zunszain PA, Thuret S, Pariante CM (2015) The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci 38(3):145–157. https://doi.org/10.1016/j.tins.2014.12.006
Lopes RS, Cardoso MM, Sampaio AO, Barbosa MS Jr, Souza CC, MC DAS, Ferreira EM, Freire MA et al (2016) Indomethacin treatment reduces microglia activation and increases numbers of neuroblasts in the subventricular zone and ischaemic striatum after focal ischaemia. J Biosci 41(3):381–394
Inta D, Lang UE, Borgwardt S, Meyer-Lindenberg A, Gass P (2017) Microglia activation and schizophrenia: lessons from the effects of minocycline on postnatal neurogenesis, Neuronal Survival and Synaptic Pruning. Schizophr Bull 43(3):493–496. https://doi.org/10.1093/schbul/sbw088
Sellner S, Paricio-Montesinos R, Spiess A, Masuch A, Erny D, Harsan LA, Elverfeldt DV, Schwabenland M et al (2016) Microglial CX3CR1 promotes adult neurogenesis by inhibiting Sirt 1/p65 signaling independent of CX3CL1. Acta Neuropathol Commun 4(1):102. https://doi.org/10.1186/s40478-016-0374-8
Zhou H, Lapointe BM, Clark SR, Zbytnuik L, Kubes P (2006) A requirement for microglial TLR4 in leukocyte recruitment into brain in response to lipopolysaccharide. J Immunol 177(11):8103–8110
Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G et al (2013) Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature 497(7448):211–216. https://doi.org/10.1038/nature12143
Yang R, Chen W, Lu Y, Li Y, Du H, Gao S, Dong X, Yuan H (2017) Dioscin relieves endotoxemia induced acute neuro-inflammation and protect neurogenesis via improving 5-HT metabolism. Sci Rep 7:40035. https://doi.org/10.1038/srep40035
Gao M, Chen L, Yu H, Sun Q, Kou J, Yu B (2013) Diosgenin down-regulates NF-kappaB p65/p50 and p38MAPK pathways and attenuates acute lung injury induced by lipopolysaccharide in mice. Int Immunopharmacol 15(2):240–245. https://doi.org/10.1016/j.intimp.2012.11.019
Hirai S, Uemura T, Mizoguchi N, Lee JY, Taketani K, Nakano Y, Hoshino S, Tsuge N et al (2010) Diosgenin attenuates inflammatory changes in the interaction between adipocytes and macrophages. Mol Nutr Food Res 54(6):797–804. https://doi.org/10.1002/mnfr.200900208
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2018R1D1A3B07051424, 2018R1A6A3A11040439, 2018R1D1A1B07049876) and by a grant from the Chonnam National University Hospital Research Institute of Clinical Medicine (CRI 12052-22).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Fig. S1
(A) Chemical structures of Arg-DG and (B) schematic of the experimental design. Mice were randomly divided into three groups (1, sham control; 2, Arg-DG treatment, LPS intracerebroventricular (I.C.V) injection; and LPS I.C.V. injection with Arg-DG co-treatment) and received 3 μg of LPS with I.C.V. injection. Arg-DG solution (0.6 mg/kg) dissolved in saline was intraperitoneally administrated (IP) three times at 8-h intervals after recovering from anesthesia. 5-Bromo-2-deoxyuridine (BrdU) was intraperitoneally injected at the indicated time points. Animals were sacrificed at the indicated date presented in the experimental schedule. (C) The proliferation of adult NSCs in the hippocampal DG was not affected by Arg-DG or PBS I.C.V. injection compared to sham control, as determined by the numbers of BrdU-positive cells using immunohistochemical analysis. (PNG 736 kb)
ESM 1
(TIF 20504 kb)
Rights and permissions
About this article
Cite this article
Cai, B., Seong, KJ., Bae, SW. et al. Water-Soluble Arginyl–Diosgenin Analog Attenuates Hippocampal Neurogenesis Impairment Through Blocking Microglial Activation Underlying NF-κB and JNK MAPK Signaling in Adult Mice Challenged by LPS. Mol Neurobiol 56, 6218–6238 (2019). https://doi.org/10.1007/s12035-019-1496-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-1496-3