Abstract
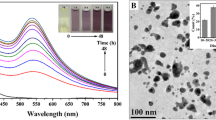
When cells of Schwanniomyces occidentalis NCIM 3459 were incubated with 1 mM tetrachloroauric acid (HAuCl4) or silver nitrate (AgNO3), cell-associated nanoparticles were obtained. Their presence was confirmed by scanning electron microscope observations. The cell-free supernatant (CFS) of the yeast mediated the synthesis of gold nanoparticles. On account of the difficulties associated with the use of cell-bound nanoparticles, further work was restricted to extracellular nanoparticles. It was hypothesized that the CFS contained thermostable biomolecule(s) that mediated metal reduction reactions. Extraction of the CFS with chloroform/methanol (2:1) and subsequent separation by preparative thin layer chromatography led to the activity-guided purification of a glycolipid. The glycolipid was hydrolyzed and the glycone (glucose) and aglycone components (palmitic acid and oleic acid) were identified by gas chromatography-mass spectrometry. The purified glycolipid mediated the synthesis of gold and silver nanoparticles that were characterized by using an X-ray diffractometer and transmission electron microscope (TEM). The extracellular nanoparticles displayed catalytic activities and reduced 4-nitroaniline to benzene-1,4-diamine. This paper thus highlights nanoparticle synthesis by a hitherto unreported yeast culture, identifies the biomolecules involved in the process, and describes a potential application of the nanostructures.








Similar content being viewed by others
References
Chauhan, A., Zubair, S., Tufail, S., Sherwani, A., Sajid, M., Raman, S. C., Azam, A., & Owais, M. (2011). Fungus-mediated biological synthesis of gold nanoparticles: potential in detection of liver cancer. International Journal of Nanomedicine, 6, 2305–2319.
Saha, K., Agasti, S. S., Kim, C., Li, X., & Rotello, V. M. (2012). Gold nanoparticles in chemical and biological sensing. Chemical Reviews, 112, 2739–2779.
Wang, W., Pang, Y., Yan, J., Wang, G., Suo, H., Zhao, C., & Xing, S. (2012). Facile synthesis of hollow urchin-like gold nanoparticles and their catalytic activity. Gold Bulletin, 45, 91–98.
Rodrigues, A. G., Ping, L. Y., Marcato, P. D., Alves, O. L., Silva, M. C. P., Ruiz, R. C., Melo, I. S., Tasic, L., & De Souza, A. O. (2013). Biogenic antimicrobial silver nanoparticles produced by fungi. Applied Microbiology and Biotechnology, 97, 775–782.
Golinska, P., Wypij, M., Ingle, A. P., Gupta, I., Dahm, H., & Rai, M. (2014). Biogenic synthesis of metal nanoparticles from actinomycetes: biomedical applications and cytotoxicities. Applied Microbiology and Biotechnology, 98, 8083–8097.
Singh, R., Shedbalkar, U. U., Wadhwani, S. A., & Chopade, B. A. (2015). Bacteriagenic silver nanoparticles: synthesis, mechanism, and applications. Applied Microbiology and Biotechnology, 99, 4579–4593.
K, S., Vaghasiya, J. V., Soni, S. S., Patel, J., Patel, R., Kumari, M., Jasmani, F., & Selvaraj, K. (2015). Microbial selenium nanoparticles (SeNPs) and their application as a sensitive hydrogen peroxide biosensor. Applied Biochemistry and Biotechnology, 177, 1386–1393.
Moorthi, P. V., Balasubramanian, C., & Mohan, S. (2015). An improved insecticidal activity of silver nanoparticle synthesized by using Sargassum muticum. Applied Biochemistry and Biotechnology, 175, 135–140.
Park, T. J., Lee, K. G., & Lee, S. Y. (2016). Advances in microbial biosynthesis of metal nanoparticles. Appl Microbiol Biotechnol, 100, 521–534.
Li, Z., & Du, Y. (2003). Biomimic synthesis of CdS nanoparticles with enhanced luminescence. Materials Letters, 57, 2480–2484.
Jha, A. K., Prasad, K., & Prasad, K. (2009). A green low cost biosynthesis of Sb2O3 nanoparticles. Biochemical Engineering Journal, 43, 303–306.
Jha, A. K., Prasad, K., & Kulkarni, A. R. (2009). Synthesis of TiO2 nanoparticles using microorganisms. Colloids Surfaces B, 71, 226–229.
Kowshik, M., Deshmukh, N., Vogel, W., Urban, J., Kulkarni, S. K., & Paknikar, K. M. (2002). Microbial synthesis of semiconductor CdS nanoparticles, their characterization, and their use in the fabrication of an ideal diode. Biotechnology and Bioengineering, 78, 583–588.
Gericke, M., & Pinches, A. (2006). Microbial production of gold nanoparticles. Gold Bulletin, 39, 1.
Subramanian, M., Alikunhi, N. M., & Kandasamy, K. (2010). In vitro synthesis of silver nanoparticles by marine yeasts from coastal mangrove sediment. Advanced Science Letters, 3, 428–433.
Kumar, G., Mamidyala, S., Das, B., Sridhar, B., Sarala Devi, V., & Karuna, M. (2010). Synthesis of biosurfactant-based silver nanoparticles with purified rhamnolipids isolated from Pseudomonas aeruginosa BS-161R. Biotechnology, 20, 1061–1068.
Zinjarde, S., Apte, M., Mohite, P., & Kumar, A. R. (2014). Yarrowia lipolytica and pollutants: interactions and applications. Biotechnology Advances, 32, 920–933.
Kowshik, M., Vogel, W., Urban, J., Kulkarni, S. K., & Paknikar, K. M. (2002). Microbial synthesis of semiconductor PbS nanocrystallites. Advanced Materials, 14, 815–818.
Seshadri, S., Saranya, K., & Kowshik, M. (2011). Green synthesis of lead sulfide nanoparticles by the lead resistant marine yeast, Rhodosporidium diobovatum. Biotechnology Progress, 27, 1464–1469.
Durán, N., Marcato, P. D., Durán, M., Yadav, A., Gade, A., & Rai, M. (2011). Mechanistic aspects in the biogenic synthesis of extracellular metal nanoparticles by peptides, bacteria, fungi, and plants. Applied Microbiology and Biotechnology, 90, 1609–1624.
Yadav, A., Kon, K., Kratosova, G., Duran, N., Ingle, A. P., & Rai, M. (2015). Fungi as an efficient mycosystem for the synthesis of metal nanoparticles: progress and key aspects of research. Biotechnology Letters, 37, 2099–2120.
Hosseini-Abari, A., Emtiazi, G., Lee, S. H., Kim, B. G., & Kim, J. H. (2014). Biosynthesis of silver nanoparticles by Bacillus stratosphericus spores and the role of Dipicolinic Acid in this process. Applied Biochemistry and Biotechnology, 174, 270–282.
Mashwani, Z. R., Khan, T., Khan, M. A., & Nadhman, A. (2015). Synthesis in plants and plant extracts of silver nanoparticles with potent antimicrobial properties: current status and future prospects. Applied Microbiology and Biotechnology. doi:10.1007/s00253-015-6987-1.
Apte, M., Girme, G., Nair, R., Bankar, A., Kumar, A. R., & Zinjarde, S. (2013). Melanin mediated synthesis of gold nanoparticles by Yarrowia lipolytica. Materials Letters, 95, 149–152.
Dohmen, R. J., & Hollenberg, C. P. (1996). Schwanniomyces occidentalis. In K. Wolf (Ed.), Non-conventional yeasts in biotechnology (pp. 117–137). New York: Springer.
Abarca, D., Fernández-Lobato, M., del Pozo, L., & Jiménez, A. (1991). Isolation of a new gene (SW A2) encoding an α-amylase from Schwanniomyces occidentalis and its expression in Saccharomyces cerevisiae. FEBS Letters, 279, 41–44.
Álvaro-Benito, M., Polo, A., González, B., Fernández-Lobato, M., & Sanz-Aparicio, J. (2010). Structural and kinetic analysis of Schwanniomyces occidentalis invertase reveals a new oligomerization pattern and the role of its supplementary domain in substrate binding. Journal of Biological Chemistry, 285, 13930–13941.
Horn, C. H., Du Preez, J. C., & Kilian, S. G. (1992). Fermentation of grain sorghum starch by co-cultivation of Schwanniomyces occidentalis and Saccharomyces cerevisiae. Bioresource Technology, 42, 27–31.
Anupama, & Ravindra, P. (2000). Value-added food: single cell protein. Biotechnology Advances, 8, 459–479.
Fedorovych, D., Kszeminska, H., Babjak, L., Kaszycki, P., & Kołoczek, H. (2001). Hexavalent chromium stimulation of riboflavin synthesis in flavinogenic yeast. BioMetals, 14, 23–31.
Folch, J., Lees, M., & Sloane-Stanley, G. H. (1957). A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry, 226, 497–509.
Peng, F., Liu, Z., Wang, L., & Shao, Z. (2007). An oil-degrading bacterium: Rhodococcus erythropolis strain 3C-9 and its biosurfactants. Journal of Applied Microbiology, 102, 1603–1611.
Sprott, G. D., Larocque, S., Cadotte, N., Dicaire, C. J., McGee, M., & Brisson, J. R. (2003). Novel polar lipids of halophilic eubacterium Planococcus H8 and archaeon Haloferax volcanii. Biochimie Biophysical Acta, 1633, 179–188.
Sweeley, C., & Walker, B. (1964). Determination of carbohydrates in glycolipids and gangliosides by gas chromatography. Analytical Chemistry, 36, 1461–1469.
Agnihotri, M., Joshi, S., Kumar, A. R., Zinjarde, S., & Kulkarni, S. (2009). Biosynthesis of gold nanoparticles by the tropical marine yeast Yarrowia lipolytica NCIM 3589. Materials Letters, 63, 1231–1234.
Castro Longoria, E., Vilchis Nestor, A. R., & Avalos Borja, M. (2011). Biosynthesis of silver, gold and bimetallic nanoparticles using the filamentous fungus Neurospora crassa. Colloids and Surfaces B, 83, 42–48.
Ahmad, A., Senapati, S., Khan, M. I., Kumar, R., & Sastry, M. (2005). Extra-/intracellular biosynthesis of gold nanoparticles by an alkalotolerant fungus, Trichothecium sp. Journal of Biomedical Nanotechnology, 1, 47–53.
Du, L., Xian, L., & Feng, J. X. (2011). Rapid extra-/intracellular biosynthesis of gold nanoparticles by the fungus Penicillium sp. Journal of Nanoparticle Research, 13, 921–930.
Velmurugan, P., Lee, S. M., Cho, M., Park, J. H., Seo, S. K., Myung, H., Bang, K. S., & Oh, B. T. (2014). Antibacterial activity of silver nanoparticle-coated fabric and leather against odor and skin infection causing bacteria. Applied Microbiology and Biotechnology, 98, 8179–8189.
Dusane, D., Pawar, V., Nancharaiah, Y., Venugopalan, V., Kumar, A., & Zinjarde, S. (2011). Anti-biofilm potential of a glycolipid surfactant produced by a tropical marine strain of Serratia marcescens. Biofouling, 27, 645–654.
Golmoraj, V., Khoshayand, M., Amini, M., Moghadamd, K., Amin, G., & Shahverdi, A. (2011). The surface chemistry and stability of gold nanoparticles prepared using methanol extract of Eucalyptus camaldulensis. Journal of Experimental Nanoscience, 6, 200–208.
Jain, R., Mody, K., Mishra, A., & Jha, B. (2012). Physicochemical characterization of biosurfactant and its potential to remove oil from soil and cotton cloth. Carbohydrate Polymers, 89, 1110–1116.
Inès, M., & Dhouha, G. (2015). Glycolipid biosurfactants: potential related biomedical and biotechnological applications. Carbohydrate Research, 416, 59–69.
Kurtzman, C. P., Price, N. P. J., Ray, K. J., & Kuo, T. M. (2010). Production of sophorolipid biosurfactants by multiple species of the Starmerella (Candida) bombicola yeast clade. FEMS Microbiology Letters, 311, 140–146.
Kulakovskaya, T., Shashkov, A., Kulakovskaya, E., Golubev, W., Zinin, A., Tsvetkov, Y., Grachev, A., & Nifantiev, N. (2009). Extracellular cellobiose lipid from yeast and their analogues: structures and fungicidal activities. Journal of Oleo Science, 58, 133–140.
Kulakovskaya, T. V., Golubev, W. I., Tomashevskaya, M. A., Kulakovskaya, E. V., Shashkov, A. S., Grachev, A. A., Chizhov, A. S., & Nifantiev, N. E. (2010). Production of antifungal cellobiose lipids by Trichosporon porosum. Mycopathologia, 169, 117–123.
Morita, T., Fukuoka, T., Imura, T., & Kitamoto, D. (2013). Accumulation of cellobiose lipids under nitrogen-limiting conditions by two ustilaginomycetous yeasts, Pseudozyma aphidis and Pseudozyma hubeiensis. FEMS Yeast Research, 13, 44–49.
Morita, T., Ishibashi, Y., Fukuoka, T., Imura, T., Sakai, H., Abe, M., & Kitamoto, D. (2011). Production of glycolipid biosurfactants, cellobiose lipids, by Cryptococcus humicola JCM 1461 and their interfacial properties. Bioscience Biotechnology and Biochemistry, 75, 1597–1599.
Joshi-Navare, K., Singh, P. K., & Prabhune, A. A. (2014). New yeast isolate Pichia caribbica synthesizes xylolipid biosurfactant with enhanced functionality. European Journal of Lipid Science and Technology. doi:10.1002/ejlt.201300363.
Déziel, E., Lépine, F., Milot, S., & Villemur, R. (2000). Mass spectrometry monitoring of rhamnolipids from a growing culture of Pseudomonas aeruginosa strain 57RP. Biochimica et Biophysica Acta, 1485, 145–152.
Abalos, A., Pinazo, A., Infante, M., Casals, M., Garcia, F., & Manresa, A. (2001). Physicochemical and antimicrobial properties of new rhamnolipids produced by Pseudomonas aeruginosa AT10 from soybean oil refinery wastes. Langmuir, 17, 1367–1371.
Johnson, M., & Boese-Marrazzo, D. I. (1980). Production and properties of heat-stable extracellular hemolysin from Pseudomonas aeruginosa. Infection and Immunity, 29, 1028–1033.
Häußler, S., Nimtz, M., Domke, T., Wray, V., & Steinmetz, V. (1998). Purification and characterization of a cytotoxic exolipid of Burkholderia pseudomallei. Infection and Immunity, 66, 1588–1593.
Rehman, A., Raza, Z., Saif-ur-Rehman, Khalid, Z., Subramani, C., Rotello, V., & Hussain, I. (2010). Synthesis and use of self-assembled rhamnolipid microtubules as templates for gold nanoparticles assembly to form gold microstructures. Journal of Colloid and Interface Science, 347, 332–335.
Kumar, D., Karthik, S. L., Kumar, G., & Rao, K. V. B. (2011). Biosynthesis of silver nanoparticles from marine yeast and their antimicrobial activity against multidrug resistant pathogens. Pharmacologyonline, 3, 1100–1111.
Ganesh, K. C., Mamidyala, S. K., Das, B., Sridhar, B., Devi, G. S., & Karuna, M. S. (2010). Synthesis of biosurfactant-based silver nanoparticles with purified rhamnolipids isolated from Pseudomonas aeruginosa BS-161R. Journal of Microbiology and Biotechnology, 20, 1061–1068.
Leelavathi, A., Rao, T. U. B., & Pradeep, T. (2011). Supported quantum clusters of silver as enhanced catalysts for reduction. Nanoscale Research Letters, 6, 123–132.
Nalawade, P., Mukherjee, T., & Kapoor, S. (2013). Green synthesis of gold nanoparticles using glycerol as a reducing agent. Advance Nanoparticles, 2, 78–86.
Chiu, C. Y., Chung, P. J., Lao, K. U., Liao, C. W., & Huang, M. H. (2012). Facet-dependent catalytic activity of gold nanocubes, octahedra, and rhombic dodecahedra toward 4-nitroaniline reduction. Journal of Physical Chemistry C, 116, 23757–23763.
Liu, Y., Liu, L., Yuan, M., & Guo, R. (2013). Preparation and characterization of casein-stabilized gold nanoparticles for catalytic applications. Colloids and Surfaces A, 417, 18–25.
Acknowledgments
This work was supported by funds from University Grants Commission under University with Potential for Excellence, Phase II. PTM thanks Council of Scientific and Industrial Research (CSIR), New Delhi, India, for Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mohite, P., Apte, M., Kumar, A.R. et al. Biogenic Nanoparticles from Schwanniomyces occidentalis NCIM 3459: Mechanistic Aspects and Catalytic Applications. Appl Biochem Biotechnol 179, 583–596 (2016). https://doi.org/10.1007/s12010-016-2015-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2015-x