Abstract
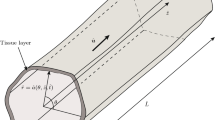
Scaffolds engineered for in vitro tissue engineering consist of multiple pores where cells can migrate along with nutrient-rich culture medium. The presence of the nutrient medium throughout the scaffold pores promotes cell proliferation, and this process depends on several factors such as scaffold geometry, nutrient medium flow rate, shear stress, cell-scaffold focal adhesions and elastic properties of the scaffold material. While numerous studies have addressed the first four factors, the mathematical approach described herein focuses on cell proliferation rate in elastic scaffolds, under constant flux of nutrients. As cells proliferate, the scaffold pores radius shrinks and thus, in order to sustain the nutrient flux, the inlet applied pressure on the upstream side of the scaffold pore must be increased. This results in expansion of the elastic scaffold pore, which in turn further increases the rate of cell proliferation. Considering the elasticity of the scaffold, the pore deformation allows further cellular growth beyond that of inelastic conditions. In this paper, our objectives are as follows: (i) Develop a mathematical model for describing fluid dynamics, scaffold elasticity and cell proliferation for scaffolds consist of identical nearly cylindrical pores; (ii) Solve the models and then simulate cellular proliferation within an elastic pore. The simulation can emulate real life tissue growth in a scaffold and offer a solution which reduces the numerical burdens. Lastly, our results demonstrated are in qualitative agreement with experimental observations reported in the literature.






Similar content being viewed by others
References
AA JV, Misra S, Fitzgerald B (2018) Acute transplantation rejection
Akhmanova M, Osidak E, Domogatsky S, Rodin S, Domogatskaya A (2015) Physical, spatial, and molecular aspects of extracellular matrix of in vivo niches and artificial scaffolds relevant to stem cells research. Stem Cells Int 2015
Anand V, Christov IC (2020) Transient compressible flow in a compliant viscoelastic tube. Phys Fluids 32(11):112014
Anand V, Christov IC (2021) Revisiting steady viscous flow of a generalized Newtonian fluid through a slender elastic tube using shell theory. Wiley Online Library, USA
Bachmann B, Spitz S, Schädl B, Teuschl AH, Redl H, Nürnberger S, Ertl P (2020) Stiffness matters: fine-tuned hydrogel elasticity alters chondrogenic redifferentiation. Front Bioeng Biotechnol 8:373
Barthes J, Özçelik H, Hindié M, Ndreu-Halili A, Hasan A, Vrana NE (2014) Cell microenvironment engineering and monitoring for tissue engineering and regenerative medicine: the recent advances. BioMed Res Inte 2014
Bidan CM, Kommareddy KP, Rumpler M, Kollmannsberger P, Fratzl P, Dunlop JW (2013) Geometry as a factor for tissue growth: towards shape optimization of tissue engineering scaffolds. Adv Healthcare Mater 2(1):186–194
Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee H-P, Lippens E, Duda GN et al (2016) Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater 15(3):326–334
Chen Z, Liu SY, Christov IC, Sanaei P (2021) Flow and fouling in elastic membrane filters with hierarchical branching pore morphology. Phys Fluids 33(6):062009
Cheng G, Markenscoff P, Zygourakis K (2009) A 3d hybrid model for tissue growth: the interplay between cell population and mass transport dynamics. Biophys J 97(2):401–414
Chung C, Chen C, Chen C, Tseng C (2007) Enhancement of cell growth in tissue-engineering constructs under direct perfusion: modeling and simulation. Biotechnol Bioeng 97(6):1603–1616
Doyle AD, Carvajal N, Jin A, Matsumoto K, Yamada KM (2015) Local 3d matrix microenvironment regulates cell migration through spatiotemporal dynamics of contractility-dependent adhesions. Nat Commun 6(1):1–15
Dunn JC, Chan W-Y, Cristini V, Kim J, Lowengrub J, Singh S, Wu BM (2006) Analysis of cell growth in three-dimensional scaffolds. Tissue Eng 12(4):705–716
Elosegui-Artola A, Oria R, Chen Y, Kosmalska A, Pérez-González C, Castro N, Zhu C, Trepat X, Roca-Cusachs P (2016) Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat Cell Biol 18(5):540–548
Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126(4):677–689
Freed L, Marquis J, Langer R, Vunjak-Novakovic G (1994) Kinetics of chondrocyte growth in cell-polymer implants. Biotechnol Bioeng 43(7):597–604
Guimarães CF, Gasperini L, Marques AP, Reis RL (2020) The stiffness of living tissues and its implications for tissue engineering. Nat Rev Mater 5(5):351–370
Howell P, Kozyreff G, Ockendon J (2009) Applied solid mechanics. Cambridge University Press, UK
Humphrey JD, Dufresne ER, Schwartz MA (2014) Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 15(12):802–812
Irawan V, Higuchi A, Ikoma T (2018) Physical cues of biomaterials guide stem cell fate of differentiation: the effect of elasticity of cell culture biomaterials. Open Phys 16(1):943–955
Janmey PA, Fletcher DA, Reinhart-King CA (2020) Stiffness sensing by cells. Physiol Rev 100(2):695–724
Jhala D, Vasita R (2015) A review on extracellular matrix mimicking strategies for an artificial stem cell niche. Polym Rev 55(4):561–595
Ji Q, Wang YL, Xia LM, Yang Y, Wang CS, Mei YQ (2019) High shear stress suppresses proliferation and migration but promotes apoptosis of endothelial cells co-cultured with vascular smooth muscle cells via down-regulating mapk pathway. J Cardiothorac Surg 14(1):1–10
Kanungo BP, Gibson LJ (2010) Density-property relationships in collagen-glycosaminoglycan scaffolds. Acta Biomater 6(2):344–353
Kennedy KM, Bhaw-Luximon A, Jhurry D (2017) Cell-matrix mechanical interaction in electrospun polymeric scaffolds for tissue engineering: implications for scaffold design and performance. Acta Biomater 50:41–55
Kirby TJ, Lammerding J (2018) Emerging views of the nucleus as a cellular mechanosensor. Nat Cell Biol 20(4):373–381
Kommareddy KP, Lange C, Rumpler M, Dunlop JW, Manjubala I, Cui J, Kratz K, Lendlein A, Fratzl P (2010) Two stages in three-dimensional in vitro growth of tissue generated by osteoblastlike cells. Biointerphases 5(2):45–52
Kumar P, Raja Sekhar G (2020) Analysis of elastohydrodynamics and nutrient transport through deformable porous scaffold inside a hollow fiber membrane bioreactor. Phys Fluids 32(3):031904
Kumar P, Sekhar GR (2020) Elastohydrodynamics of a deformable porous packing in a channel competing under shear and pressure gradient. Phys Fluids 32(6):061901
Kumar P, Dey B, Sekhar GR (2018) Nutrient transport through deformable cylindrical scaffold inside a bioreactor: an application to tissue engineering. Int J Eng Sci 127:201–216
Kwon SG, Kwon YW, Lee TW, Park GT, Kim JH (2018) Recent advances in stem cell therapeutics and tissue engineering strategies. Biomater Res 22(1):1–8
Lee-Thedieck C, Spatz JP (2014) Biophysical regulation of hematopoietic stem cells. Biomater Sci 2(11):1548–1561
Lemon G, King J (2007) Multiphase modelling of cell behaviour on artificial scaffolds: effects of nutrient depletion and spatially nonuniform porosity. Math Med Biol 24(1):57–83
Lemon G, King JR, Byrne HM, Jensen OE, Shakesheff KM (2006) Mathematical modelling of engineered tissue growth using a multiphase porous flow mixture theory. J Math Biol 52(5):571–594
Levy-Mishali M, Zoldan J, Levenberg S (2009) Effect of scaffold stiffness on myoblast differentiation. Tissue Eng Part A 15(4):935–944
Malda J, Rouwkema J, Martens D, le Comte EP, Kooy F, Tramper J, Van Blitterswijk C, Riesle J (2004) Oxygen gradients in tissue-engineered PEGT/PBT cartilaginous constructs: measurement and modeling. Biotechnol Bioeng 86(1):9–18
Malvè M, Bergstrom D, Chen X (2018) Modeling the flow and mass transport in a mechanically stimulated parametric porous scaffold under fluid-structure interaction approach. Int Commun Heat Mass Transfer 96:53–60
Marion NW, Mao JJ (2006) Mesenchymal stem cells and tissue engineering. Methods Enzymol 420:339–361
Martino F, Perestrelo AR, Vinarskỳ V, Pagliari S, Forte G (2018) Cellular mechanotransduction: from tension to function. Front Physiol 9:824
McKee CT, Last JA, Russell P, Murphy CJ (2011) Indentation versus tensile measurements of young’s modulus for soft biological tissues. Tissue Eng Part B Rev 17(3):155–164
Nagano M, Hoshino D, Koshikawa N, Akizawa T, Seiki M (2012) Turnover of focal adhesions and cancer cell migration. Int J Cell Biol 2012
Nelson CM, Gleghorn JP (2012) Sculpting organs: mechanical regulation of tissue development. Annu Rev Biomed Eng 14:129–154
Nelson CM, Jean RP, Tan JL, Liu WF, Sniadecki NJ, Spector AA, Chen CS (2005) Emergent patterns of growth controlled by multicellular form and mechanics. Proc Natl Acad Sci 102(33):11594–11599
O’Brien FJ, Harley B, Yannas IV, Gibson LJ (2005) The effect of pore size on cell adhesion in collagen-gag scaffolds. Biomaterials 26(4):433–441
O’Brien FJ, Harley B, Yannas IV, Gibson LJ (2005) The effect of pore size on cell adhesion in collagen-gag scaffolds. Biomaterials 26(4):433–441
O’Dea RD, Waters SL, Byrne HM (2010) A multiphase model for tissue construct growth in a perfusion bioreactor. Math Med Biol J IMA 27(2):95–127
Pearson NC, Shipley RJ, Waters SL, Oliver JM (2014) Multiphase modelling of the influence of fluid flow and chemical concentration on tissue growth in a hollow fibre membrane bioreactor. Math Med Biol J IMA 31(4):393–430
Prasadh S, Wong RCW (2018) Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Sci Int 15(2):48–55
Putnam AJ, Cunningham JJ, Dennis RG, Linderman JJ, Mooney DJ (1998) Microtubule assembly is regulated by externally applied strain in cultured smooth muscle cells. J Cell Sci 111(22):3379–3387
Rouwkema J, Koopman BF, Blitterswijk CAV, Dhert WJ, Malda J (2009) Supply of nutrients to cells in engineered tissues. Biotechnol Genet Eng Rev 26(1):163–178
Rowlands AS, George PA, Cooper-White JJ (2008) Directing osteogenic and myogenic differentiation of MSCS: interplay of stiffness and adhesive ligand presentation. Am J Physiol Cell Physiol 295(4):1037–1044
Rumpler M, Woesz A, Dunlop JW, Van Dongen JT, Fratzl P (2008) The effect of geometry on three-dimensional tissue growth. J R Soc Interface 5(27):1173–1180
Sanaei P, Cummings LJ (2018) Membrane filtration with complex branching pore morphology. Phys Rev Fluids 3(9):094305
Sanaei P, Cummings L, Waters S, Griffiths I (2019) Curvature-and fluid-stress-driven tissue growth in a tissue-engineering scaffold pore. Biomech Model Mechanobiol 18(3):589–605
Shakeel M, Matthews PC, Graham RS, Waters SL (2013) A continuum model of cell proliferation and nutrient transport in a perfusion bioreactor. Math Med Biol J IMA 30(1):21–44
Shipley RJ, Waters SL (2012) Fluid and mass transport modelling to drive the design of cell-packed hollow fibre bioreactors for tissue engineering applications. Math Med Biol J IMA 29(4):329–359
Shkumatov A, Thompson M, Choi KM, Sicard D, Baek K, Kim DH, Tschumperlin DJ, Prakash Y, Kong H (2015) Matrix stiffness-modulated proliferation and secretory function of the airway smooth muscle cells. Am J Physiol-Lung Cell Molecular Physiol 308(11):1125–1135
Truesdell C, Noll W (2004) The non-linear field theories of mechanics. Springer, USA
Ueba H, Kawakami M, Yaginuma T (1997) Shear stress as an inhibitor of vascular smooth muscle cell proliferation: role of transforming growth factor-\(\beta \)1 and tissue-type plasminogen activator. Arterioscler Thromb Vasc Biol 17(8):1512–1516
Wei-hui G, Frey MT, Burnham NA, Yu-li W (2006) Substrate rigidity regulates the formation and maintenance of tissues. Biophys J 90(6):2213–20
Yannas IV (2014) Tissue and organ regeneration in adults: extension of the paradigm to several organs
Yeh Y-T, Hur SS, Chang J, Wang K-C, Chiu J-J, Li Y-S, Chien S (2012) Matrix stiffness regulates endothelial cell proliferation through septin 9. PLoS ONE 7(10):46889
Zong Z, Li X, Sanaei P (2021) Effects of nutrient depletion on tissue growth in a tissue engineering scaffold pore. Phys Fluids 33(12):121903
Acknowledgements
C.A. and P.S. are supported by an Institutional Support of Research and Creativity (ISRC) grant provided by New York Institute of Technology. H.F. and P.S. acknowledge financial support from Molecular Basis of Disease PhD Fellowship at Georgia State University and the National Science Foundation (NSF) under Grants No. DMS-2108161.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The views expressed in this article are D. Fong’s own and not those of the U.S. Merchant Marine Academy, the Maritime Administration, the Department of Transportation, or the United States government.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 1 (mp4 2253 KB)
Appendices
Appendix A
In this appendix, we justify the assumption of the elasto-static condition described in Sect. 2.2. With the assumptions mentioned in Sect. 2.2, elastic pore deformation is described by the Navier–Cauchy equations as
where the displacement vector and the density of the elastic pore are represented by \(\varvec{\Omega }=(\Omega _r,\Omega _\theta ,\Omega _z)\) and \(\rho _m\), respectively. Young’s Modulus E, and Poisson’s ratio \(\nu \) are used to quantify the compliance of the elastic construct. After applying the scalings given in (14), (24), (30) and dropping \(\hat{}\), (A1) simplifies to
where \(\varvec{\Omega }=(\Omega _r,\epsilon \Omega _\theta ,\Omega _z)\) is now the dimensionless displacement vector. The coefficient \({\frac{2(1+\nu )\rho _m \lambda ^2}{E R^2}\ll 1}\) according to the parameters’ values given in Table 1. Therefore, in the regime we are focusing on in this paper, the term \(\frac{\partial ^2\varvec{\Omega }}{\partial t^2}\) can be set to zero, which is the elasto-static condition.
Appendix B
In this appendix, we present the detailed calculations of (45)-(48). We solve the solution for \(a_0(z,t)\) and \(\Lambda (z,t)\) by treating each (time) interval separately, and match the solutions in the different intervals by requiring that a(z, t) and \(\Lambda (z,t)\) be continuous functions of t. The first part of (44), which is \(\frac{\partial a_0(z,t)}{\partial t} = - \kappa _0 f+\frac{\partial \Omega _{r_0}(z,t)}{\partial t}\), can be solved as below. We begin by plugging f and \( \Omega _{r_0}(z,t)\) from (32) and (43), respectively, into the first part of (44) to get
Next, we multiply both sides of the first part of (B3) by \(a_0\) and simplify the right hand side of the equation to obtain
Integrating both sides of (B4) with respect to t gives
where \(c_1\) can be determined by setting \(t=0\) in (B5) as
Substituting \(c_1\) into (B5) and multiplying both sides by \(2a_0^2(z,t)\) gives a quadratic equation for \(a_0^2(z,t)\):
Solving (B7) for \(a_0(z,t)\) in the time interval \(0 \le t < t_{01}\) yields
To find \(t_{01}\), we set \(\frac{4}{a_0^3(z,t_{01})}=\sigma _1\) and it is convenient to write it in the equivalent form
By evaluating (B8) for \(t=t_{01}\) and using (B9), after some algebraic manipulations, we obtain
In order to solve the second part of (B3), we will follow a process similar to what we have done for the first part of (B3). Therefore, the second part of (B3) gives
Similarly, we obtain \(c_2\) by setting \(t=t_{01}\). Substituting \(c_2\) into (B10) and using \(a_0(z,t_{01})=\left( \frac{4}{\sigma _1}\right) ^\frac{1}{3}\) together, the solution for \(a_0(z,t)\) within the time interval \(t_{01} \le t < t_{02}\) is
Setting \(t=t_{02}\) and using \(\frac{4}{{a_0^3(z,t_{02})}}=\sigma _2\) in (B11), after some manipulations, we find
Similar to the first and second parts of (B3), the third one gives
where \(c_3\) should be determined. Since \(a_0(z,t_{02})=(\frac{4}{\sigma _2})^{1/3}\), we find
and after some manipulations, we obtain
For the rest of this appendix, we show how to obtain (46). Using (32) and (39) in (44) yields
The first part of (B15) can be rewritten as
By integrating both sides of (B16) with respect to t, we obtain
where \(c_4\) is an constant of integration and should be determined. After removing the absolute value bars in (B17), and by taking the exponential of both sides, we find that
where \(c_5 = e^{c_4}\). In order to satisfy the initial condition \(\Lambda (z,0)\), we must choose \(c_5 = \Lambda (z,0)/a_0^{n^2}(z,0)\). Using this value for \(c_5\) in (B18), we obtain
The next two parts of (B15) could be obtained in a similar fashion.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Annunziata, C., Fattahpour, H., Fong, D. et al. Effects of Elasticity on Cell Proliferation in a Tissue-Engineering Scaffold Pore. Bull Math Biol 85, 25 (2023). https://doi.org/10.1007/s11538-023-01134-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-023-01134-7