Abstract
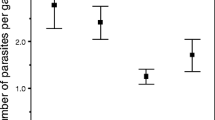
Phenotypic differences between infected and non-infected hosts are often assumed to be the consequence of parasite infection. However, pre-existing differences in hosts’ phenotypes may promote differential susceptibility to infection. The phenotypic variability observed within the host population may therefore be a cause rather than a consequence of infection. In this study, we aimed at disentangling the causes and the consequences of parasite infection by calculating the value of a phenotypic trait (i.e., the growth rate) of the hosts both before and after infection occurred. That procedure was applied to two natural systems of host–parasite interactions. In the first system, the infection level of an ectoparasite (Tracheliastes polycolpus) decreases the growth rate of its fish host (the rostrum dace, Leuciscus leuciscus). Reciprocally, this same phenotypic trait before infection modulated the future level of host sensitivity to the direct pathogenic effect of the parasite, namely the level of fin degradation. In the second model, causes and consequences linked the growth rate of the fish host (the rainbow smelt, Osmerus mordax) and the level of endoparasite infection (Proteocephalus tetrastomus). Indeed, the host’s growth rate before infection determined the number of parasites later in life, and the parasite biovolume then decreased the host’s growth rate of heavily infected hosts. We demonstrated that reciprocal effects between host phenotypes and parasite infection can occur simultaneously in the wild, and that the observed variation in the host phenotype population was not necessarily a consequence of parasite infection. Disentangling the causality of host–parasite interactions should contribute substantially to evaluating the role of parasites in ecological and evolutionary processes.




Similar content being viewed by others
References
Albon SD, Stien A, Irvine RJ, Langvatn R, Ropstad E, Halvorsen O (2002) The role of parasites in the dynamics of a reindeer population. P Roy Soc B-Biol Sci 269:1625–1632
Arbuckle JL (2003) Amos for Windows. Analysis of moment structures (version 5). SmallWaters, Chicago. http://www.smallwaters.com/amos/
Arnott SA, Barber I, Huntingford FA (2000) Parasite-associated growth enhancement in a fish-cestode system. Proc R Soc Lond B Biol Sci 267:657–663
Barber I (2005) Parasites grow larger in faster growing fish hosts. Int J Parasitol 35:137–143
Barber I, Svensson PA (2003) Effects of experimental Schistocephalus solidus infections on growth, morphology and sexual development of female three-spined sticklebacks, Gasterosteus aculeatus. Parasitology 126:359–367
Barber I, Hoare D, Krause J (2000) Effects of parasites on fish behaviour: a review and evolutionary perspective. Rev Fish Biol Fish 10:131–165
Bell AM, Sih A (2007) Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecol Lett 10:828–834. doi:810.1111/j.1461-0248.2007.01081.x
Bize P, Roulin A, Bersier LF, Pfluger D, Richner H (2003) Parasitism and developmental plasticity in Alpine swift nestlings. J Anim Ecol 72:633–639
Blair L, Webster JP (2007) Dose-dependent schistosome-induced mortality and morbidity risk elevates host reproductive effort. J Evol Biol 20:54–61. doi:10.1111/j.1420-9101.2006.01230.x
Bourque JF, Dodson JJ, Ryan DAJ, Marcogliese DJ (2006) Cestode parasitism as a regulator of early life-history survival in an estuarine population of rainbow smelt Osmerus mordax. Mar Ecol Prog Ser 314:295–307
Campana SE, Jones CM (1992) Analysis of otolith microstructure data. In: Stevenson DK, Campana SE (eds) Otolith microstructure examination and analysis. Canadian Special Publication in Fish and Aquatic Sciences, Ottawa, pp 73–100
Combes C (1991) Ethological aspect of parasite transmission. Am Nat 138:866–880
Combes C (1998) Parasitism. The ecology and evolution of intimate interactions. University of Chicago Press, Chicago
Finley RJ, Forrester GE (2003) Impact of ectoparasites on the demography of a small reef fish. Mar Ecol Prog Ser 248:305–309
Francis R (1990) Back-calculation of fish length. A critical review. J Fish Biol 36:883–902
Hall SR, Sivars-Becker L, Becker C, Duffy MA, Tessier AJ, Caceres CE (2007) Eating yourself sick: transmission of disease as a function of foraging ecology. Ecol Lett 10:207–218. doi:210.1111/j.1461-0248.2007.01011.x
Hatcher MJ, Dick JTA, Dunn AM (2006) How parasites affect interactions between competitors and predators. Ecol Lett 9:1253–1271
Holmstad PR, Jensen KH, Skorping A (2006) Vector-borne parasites decrease host mobility: a field test of freeze or flee behaviour of willow ptarmigan. Int J Parasitol 36:735–740
Hurd H (2001) Host fecundity reduction: a strategy for damage limitation? Trends Parasitol 17:363–368
Hutchings MR, Gordon IJ, Kyriazakis I, Robertson E, Jackson F (2002) Grazing in heterogeneous environments: infra- and supra-parasite distributions determine herbivore grazing decisions. Oecologia 132:453–460
Johnson JB, Omland KS (2004) Model selection in ecology and evolution. Trends Ecol Evol 19:101–108
Krasnov BR, Morand S, Hawlena H, Khokhlova IS, Shenbrot GI (2005) Sex-biased parasitism, seasonality and sexual size dimorphism in desert rodents. Oecologia 146:209–217
Lambrechts L, Fellous S, Koella JC (2006) Coevolutionary interactions between host and parasite genotypes. Trends Parasitol 22:12–16
Lauder GV, Drucker EG (2002) Forces, fishes, and fluids: hydrodynamic mechanisms of aquatic locomotion. News Physiol Sci 17:235–240
Lello J, Boag B, Hudson PJ (2005) The effect of single and concomitant pathogen infections on condition and fecundity of the wild rabbit (Oryctolagus cuniculus). Int J Parasitol 35:1509–1515
Loot G, Poulin R, Lek S, Guegan JF (2002) The differential effects of Ligula intestinalis (L.) plerocercoids on host growth in three natural populations of roach, Rutilus rutilus (L.). Ecol Freshw Fish 11:168–177
Loot G, Poulet N, Reyjol Y, Blanchet S, Lek S (2004) The effects of the ectoparasite Tracheliastes polycolpus (Copepoda: Lernaeopodidae) on the fins of rostrum dace (Leuciscus leuciscus burdigalensis). Parasitol Res 94:16–23
Marcogliese DJ, Cone DK (1997) Food webs: a plea for parasites. Trends Ecol Evol 12:320–325
Matthews WJ (1998) Patterns in freshwater fish ecology. Chapman & Hall, New York
Minchella DJ (1985) Host life-history variation in response to parasitism. Parasitology 90:205–216
Moore J, Gotelli NJ (1990) A phylogenetic perspective on the evolution of altered host behaviours: a critical look at the manipulation hypothesis. In: Barnard CJ, Behnke JM (eds) Parasitism and host behaviour. Taylor and Francis, London, pp 193–233
Nilsson JA (2003) Ectoparasitism in marsh tits: costs and functional explanations. Behav Ecol 14:175–181
Olendorf R, Rodd FH, Punzalan D, Houde AE, Hurt C, Reznick DN, Hughes KA (2006) Frequency-dependent survival in natural guppy populations. Nature 441:633–636
Paterson S, Wilson K, Pemberton JM (1998) Major histocompatibility complex variation associated with juvenile survival and parasite resistance in a large unmanaged ungulate population (Ovis aries L.). Proc Natl Acad Sci USA 95:3714–3719
Poulin R (1998) Evolutionary ecology of parasites: from individuals to communities. Chapman & Hall, London
Poulin R, Marshall LJ, Spencer HG (2000) Metazoan parasite species richness and genetic variation among freshwater fish species: cause or consequence? Int J Parasitol 30:697–703
Saino N, Calza S, Moller AP (1998) Effects of a dipteran ectoparasite on immune response and growth trade-offs in barn swallow, Hirundo rustica, nestlings. Oikos 81:217–228
Scholz T, Marcogliese DJ, Bourque JF, Škeříková A, Dodson JJ (2004) Occurrence of Proteocephalus tetrastomus (Rudolphi, 1810) (Cestoda: Proteocephalidea) in larval rainbow smelt (Osmerus mordax) in North America: identification of a potential pathogen confirmed. J Parasitol 90:425–427
Schrank AJ, Webb PW (1998) Do body and fin form affect the abilities of fish to stabilize swimming during maneuvers through vertical and horizontal tubes? Environ Biol Fish 53:365–371
Schultz ET, Topper M, Heins DC (2006) Decreased reproductive investment of female threespine stickleback Gasterosteus aculeatus infected with the cestode Schistocephalus solidus: parasite adaptation, host adaptation, or side effect? Oikos 114:303–310
Seivwright LJ, Redpath SM, Mougeot F, Leckie F, Hudson PJ (2005) Interactions between intrinsic and extrinsic mechanisms in a cyclic species: testosterone increases parasite infection in red grouse. Proc R Soc B Biol Sci 272:2299–2304
Shipley B (2000) Cause and correlation in biology: a user’s guide to path analysis. structural equations and causal inference, Cambridge University Press, New York
Sirois P, Lecomte F, Dodson JJ (1998) An otolith-based back-calculation method to account for time-varying growth rate in rainbow smelt (Osmerus mordax). Can J Fish Aquat Sci 55:2662–2671
Thomas F, Adamo S, Moore J (2005) Parasitic manipulation: where are we and where should we go? Behav Process 68:185–199
Walker PD, Abbink W, van der Velde G, Wendelaar Bonga SE (2006) A new record of Tracheliastes maculatus Kollar, 1835 (Copepoda, Siphonostomatoida, Lernaeopodidae) on common bream (Abramis brama (L., 1758)) in the Netherlands. Crustaceana 79:1015–1019
Wood CL, Byers JE, Cottingham KL, Altman I, Donahue MJ, Blakeslee AMH (2007) Parasites alter community structure. Proc Natl Acad Sci USA 104:9335–9339
Acknowledgements
We sincerely thank the many field assistants that helped in sampling fish. We also thank P. Heeb for stimulating discussions, S. Brosse and two anonymous referees for constructive comments on the manuscript and S. Kohler for statistical advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Steven Kohler.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Blanchet, S., Méjean, L., Bourque, JF. et al. Why do parasitized hosts look different? Resolving the “chicken-egg” dilemma. Oecologia 160, 37–47 (2009). https://doi.org/10.1007/s00442-008-1272-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-008-1272-y