Abstract
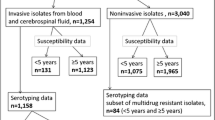
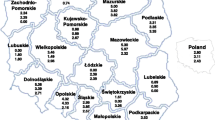
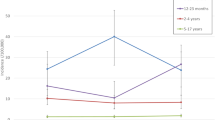
In a prospective surveillance study covering all pediatric wards in Austria, 308 cases of invasive pneumococcal disease (IPD) were reported in hospitalized children <5 years of age between 2002 and 2012. Incidence was 7.1 per 100,000 per year for IPD with a case fatality rate of 3 %, and 1.9 per 100,000 per year for pneumococcal meningitis with a case fatality rate of 9 %. At hospital discharge, 17 % of the children were not fully recovered and suffered from problems such as hearing or motor deficits. Persistent sequelae 6 months after hospital discharge were present in 13 % of the children, a finding that emphasizes the seriousness of IPD. From 2007 onwards, we observed a shift of pneumococcal serotypes from those covered by the heptavalent vaccine to serotypes consequently added to 10- and 13-valent vaccines, particularly regarding serotype 19A. Among antimicrobial resistances detected, macrolide resistance was predominant; however, between 2002 and 2012, we saw an overall decrease of resistance rates. Conclusion: Considering this change of serotypes and the high rate of permanent sequelae after IPD, our data show the importance of pediatric pneumococcal vaccination and the relevance of continuous monitoring of circulating serotypes. By the end of 2012, which was the first year of universal mass vaccination against pneumococcal disease in Austria, no change in the incidence of invasive pneumococcal disease was observed yet.


Similar content being viewed by others
Abbreviations
- IPD:
-
Invasive pneumococcal disease
- PCV7:
-
Heptavalent conjugated pneumococcal vaccines
- PCV10:
-
Ten-valent conjugated pneumococcal vaccine
- PCV13:
-
Thirteen-valent conjugated pneumococcal vaccine
- MIC:
-
Minimal inhibitory concentrations
References
Bundesanstalt Statistik Österreich. STATcube — Statistische Datenbank von Statistik Austria. Wien: Bundesanstalt Statistik Österreich 2013. http://sdb.statistik.at/superwebguest/autoLoad.do, Accessed 24 Jan 2013
Chapman KE, Wilson D, Gorton R (2013) Serotype dynamics of invasive pneumococcal disease post-PCV7 and pre-PCV13 introduction in North East England. Epidemiol Infect 141:344–352
Committee on Infectious Diseases (2000) Policy statement: recommendations for the prevention of pneumococcal infections, including the use of pneumococcal conjugate vaccine (Prevnar), pneumococcal polysaccharide vaccine, and antibiotic prophylaxis. Pediatrics 106:362–366
Dagan R (2009) Impact of pneumococcal conjugate vaccine on infections caused by antibiotic-resistant Streptococcus pneumoniae. Clin Microbiol Infect 15:16–20
Ercibengoa M, Arostegi N, Marimon J, Alonso M, Perez-Trallero E (2012) Dynamics of pneumococcal nasopharyngeal carriage in healthy children attending a day care center in northern Spain. Influence of detection techniques on the results. BMC Infect Dis 12:69
Fitzwater SP, Chandran A, Santosham M, Johnson HL (2012) The worldwide impact of the seven-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 31:501–508
Ingels H, Rasmussen J, Andersen PH, Harboe ZB, Glismann S, Konradsen H, Hoffmann S, Valentiner-Branth P, Lambertsen L (2012) Impact of pneumococcal vaccination in Denmark during the first 3 years after PCV introduction in the childhood immunization programme. Vaccine 30:3944–3950
Isaacman DJ, McIntosh ED, Reinert RR (2010) Burden of invasive pneumococcal disease and serotype distribution among Streptococcus pneumoniae isolates in young children in Europe: impact of the 7-valent pneumococcal conjugate vaccine and considerations for future conjugate vaccines. Int J Infect Dis 14:e197–e209
Kyaw MH, Lynfield R, Schaffner W, Craig AS, Hadler J, Reingold A, Thomas AR, Harrison LH, Bennett NM, Farley MM, Facklam RR, Jorgensen JH, Besser J, Zell ER, Schuchat A, Whitney CG (2006) Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med 354:1455–1463
Liu C, Xiong X, Xu W, Sun J, Wang L, Li J (2013) Serotypes and patterns of antibiotic resistance in strains causing invasive pneumococcal disease in children less than 5 Years of Age. PLoS ONE 8:e54254
Magnus MC, Vestrheim DF, Nystad W, Haberg SE, Stigum H, London SJ, Bergsaker MA, Caugant DA, Aaberge IS, Nafstad P (2012) Decline in early childhood respiratory tract infections in the Norwegian mother and child cohort study after introduction of pneumococcal conjugate vaccination. Pediatr Infect Dis J 31:951–955
Nationales Impfgremium (2012) Impfplan 2012. vol 2012. Bundeministerium für Gesundheit. http://bmg.gv.at/home/Schwerpunkte/Praevention/Impfen/Oesterreichischer_Impfplan; Accessed 22 Jul 2013
O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T (2009) Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893–902
Österreichischer Sanitätsrat. Impfplan 2010. http://www.centralapo.at/impfplan10.pdf. Accessed 6 Feb 2013
Rendi-Wagner P, Georgopoulos A, Kundi M, Mutz I, Mattauch M, Nowak J, Mikolasek A, Vecsei A, Kollaritsch H (2004) Prospective surveillance of incidence, serotypes and antimicrobial susceptibility of invasive Streptococcus pneumoniae among hospitalized children in Austria. J Antimicrob Chemother 53:826–831
Rendi-Wagner P, Paulke-Korinek M, Kundi M, Burgmann H, Georgopoulos A, Vecsei A, Kollaritsch H (2009) National paediatric immunization program of high risk groups: no effect on the incidence of invasive pneumococcal diseases. Vaccine 27:3963–3968
Resti M, Moriondo M, Cortimiglia M, Indolfi G, Canessa C, Becciolini L, Bartolini E, de Benedictis FM, de Martino M, Azzari C (2010) Community-acquired bacteremic pneumococcal pneumonia in children: diagnosis and serotyping by real-time polymerase chain reaction using blood samples. Clin Infect Dis 51:1042–1049
Rosen JB, Thomas AR, Lexau CA, Reingold A, Hadler JL, Harrison LH, Bennett NM, Schaffner W, Farley MM, Beall BW, Moore MR (2011) Geographic variation in invasive pneumococcal disease following pneumococcal conjugate vaccine introduction in the United States. Clin Infect Dis 53:137–143
Saha SK, Darmstadt GL, Baqui AH, Hossain B, Islam M, Foster D, Al-Emran H, Naheed A, Arifeen SE, Luby SP, Santosham M, Crook D (2008) Identification of serotype in culture negative pneumococcal meningitis using sequential multiplex PCR: implication for surveillance and vaccine design. PLoS ONE 3:e3576
The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. 2013; Version 3.0. http://www.eucast.org. Accessed 15 Jan 2013
van der Linden M, Weiss S, Falkenhorst G, Siedler A, Imohl M, von Kries R (2012) Four years of universal pneumococcal conjugate infant vaccination in Germany: impact on incidence of invasive pneumococcal disease and serotype distribution in children. Vaccine 30:5880–5885
Vergison A, Reinert RR (2007) Streptococcus pneumoniae incidence in Western Europe. Lancet Infect Dis 7:240–242
Weil-Olivier C, van der Linden M, de Schutter I, Dagan R, Mantovani L (2012) Prevention of pneumococcal diseases in the post-seven valent vaccine era: a European perspective. BMC Infect Dis 12:207
World Health Organization (2012) Pneumococcal vaccines WHO position paper - 2012. WER 14:129–144
Acknowledgments
Between 2009 and 2011, this study was supported by a grant of the Research Foundation of the Austrian National Bank (grant no. 13229). Between 2002 and 2008 and in 2012, this study was supported by Wyeth Lederle now Pfizer. We would like to thank Apostolos Georgopoulos who performed serotyping of S. pneumoniae between 2002 and 2007, and all pediatricians and the staff of the wards from the participating hospitals for their continued support: Univ.-Klinik f. Kinder- u. Jugendheilkunde, AKH Wien; Gottfried v. Preyer'sches Kinderspital d. Stadt Wien; Sozialmedizinisches Zentrum Ost – Donauspital; St. Anna Kinderspital Wien; Wilhelminenspital; Krankenanstalt Rudolfstiftung der Stadt Wien; Landesklinikum Baden – Mödling; Landesklinikum Tulln; LK Mistelbach – Gänserndorf; Landesklinikum Wiener Neustadt; Landesklinikum Zwettl; Landesklinikum Krems an der Donau; Landesklinikum St. Pölten; Landesklinikum Mostviertel Amstetten; Landesklinikum Scheibbs; LKH Stolzalpe; LKH Leoben; LKH - Univ. Klinikum Graz; SALK – Salzburger Universitätsklinikum; Kardinal Schwarzenberg'sches Krankenhaus; Krankenhaus St. Josef Braunau; Klinikum Wels – Grieskirchen; LKH Vöcklabruck; LKH Schärding; KH St. Franziskus; LKH Kirchdorf a. d. Krems; Landes- Frauen- und Kinderklinik Linz; Krankenhaus der Barmherzigen Schwestern Linz; LKH Steyr; KH der Bamherzigen Schwestern Ried; LKH Rohrbach; LKH Bad Ischl; Klinikum Klagenfurt am Wörthersee; LKH Villach; KH Spittal/Drau; LKH Bludenz; LKH Dornbirn; LKH Bregenz; LKH Feldkirch; Univ.klinik f. Kinder-und Jugendheilkunde, Innsbruck; Krankenhaus St. Vinzenz in Zams; BKH St. Johann in Tirol; BKH Lienz; BKH Reutte; Bezirkskrankenhaus Kufstein; KH der Barmherzigen Brüder Eisenstadt; KH Oberwart; Sozial Medizinisches Zentrum Süd – KFJ; IMED Wien; Labor Dr. Kosak; LK Mistelbach – Gänserndorf; Landesklinikum Horn; IMED Graz; Mikrobiologisches Labor Dr. Kochanowski, Dr. Mattes; KH der Elisabethinen Linz; LKH Steyr; Labor Schubach; Landesnervenklinik Wagner-Jauregg; IMED Klagenfurt; MVZ Labor Dr. Gärtner & Kollegen; Inst. f. Hygiene und Sozialmedizin, Innsbruck; and KH der Barmherzigen Brüder Eisenstadt.
Conflict of interest
Maria Paulke-Korinek accepted fees for speaking and serving on advisory boards and received funding to attend conferences from Pfizer and GlaxoSmithKline. Herwig Kollaritsch accepted educational grant fees and payment for lectures, for serving on advisory boards and as an independent safety monitor in clinical studies, and reimbursement for attending meetings from GlaxoSmithKline and Pfizer. Michael Kundi received funding for participating in advisory boards and for consultancy from Wyeth Lederle now Pfizer. Ursula Wiedermann has received grant money for investigator-initiated studies by Baxter and educational grant money by Pfizer and Novartis. Heinz Burgmann accepted fees for speaking, educational grant fees, and serving on advisory boards from Wyeth Lederle, now Pfizer. The authors have no other relevant affiliations or financial involvement with the subject matter or materials discussed in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paulke-Korinek, M., Kollaritsch, H., Kundi, M. et al. Characteristics of invasive pneumococcal disease in hospitalized children in Austria. Eur J Pediatr 173, 469–476 (2014). https://doi.org/10.1007/s00431-013-2193-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-013-2193-2