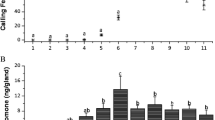
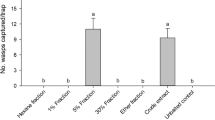
Abstract
The sexual behavior of the ectoparasitoidDiglyphus isaea is described. Recognition of the female by the male occurs at close range. Males initiate courtship behavior in the presence of a living female regardless of age, as well as in the presence of a female killed by freezing. Courtship behavior is not observed in the presence of a dead female washed with organic solvents but could be elicited using a lure covered with a female organic extract. These findings demonstrate that each sex develops a specific chemical signature that can be dissolved in hexane and transferred to a lure. Analysis of organic extracts by gas chromatography revealed chemical dimorphism between the two sexes. Gas chromatography coupled with mass spectrometry showed that the main components in females were esters of medium-chain fatty acids and long-chain 11-alcohols. There were few hydrocarbons. Female esters, which were present in only small proportions in males, were recovered in the nonhydrocarbon fraction obtained after fractionation of the total extract on a silica-filled microcolumn as a mixture containing 11-heneicosyl, 11-docosyl, 11-tricosyl, 11-tetracosyl, and 11-pentacosyl octanoate, and 11-docosyl, 11-tricosyl, 11-tetracosyl, and 11-pentacosyl decanoate. These results demonstrate that there is a specific gender-related chemical signature.
Similar content being viewed by others
References
Antony, C., andJallon, J. M. 1982. The chemical basis for sex recognition inDrosophila melanogaster.J. Insect Physiol. 28:873–880.
Attygale, A. B., Vostrowsky, O., Bestmann, H. J., andMorgan, E. D. 1987. New chemicals from the Dufour gland of the formicine antLasius niger (Hymenoptera: Formicidae).Insect Biochem. 17:219–225.
Backer, J. E., Nelson, D. R., andFatland, C. L. 1979. Developmental changes in cuticular lipids of the black carpet beetle,Attagenus megatoma.Insect Biochem. 9:335–339.
Bagnères, A. G., Killian, A., Clement, J. L., andLange, C. 1991. Interspecific recognition among termites of the genusReticulitermes: Evidence for a role for the cuticular hydrocarbons.J. Chem. Ecol. 17:2397–2420.
Bagnères, A. G., Lorenzi, M. C., Dusticier, G., Turillazzi, S., andClement, J. L. 1996. Chemical usurpation of a nest by paper wasp parasites.Science 272:889–892.
Blomquist, G. J., andJackson, L. L. 1973. Hydroxylation ofn-alkanes to secondary alcohols and their esterification in the grasshopperMelanoplus sanguinipes.Biochem. Biophys. Res. Commun. 53:703–708.
Blomquist, G. J., andJackson, L. L. 1979. Chemistry and biochemistry of insect waxes.Prog. Lipid Res. 17:319–345.
Blomquist, G. J., Soliday, C. L., Byers, B. A., Brakke, J. W., andJackson, L. L. 1972. Cuticular lipids of insects. V. Cuticular wax esters of secondary alcohols from the grasshoppersMelanoplus packardii andMelanoplus sanguinipes.Lipids 7:356–362.
Bonavita-Cougourdan, A., Clement, J. L., andLange, C. 1987. Nesmate recognition: The role of cuticular hydrocarbons in the antCamponotus vagus Scop.J. Entomol. Sci. 22:1–10.
Buckner, J. S. 1993. Cuticular polar lipids of insects, pp. 227–270,in D. W. Stanley-Samuelson and D. R. Nelson (eds.). Insect Lipids: Chemistry, Biochemistry and Biology. University of Nebraska Press, Lincoln.
Carlson, D. A., Langley, P. A., andHuyton, P. M. 1978. Sex pheromone of the tsetse fly: Isolation, identification and synthesis of contact aphrodisiacs.Science 201:750–753.
Cheah, C. S. J. 1987. Temperature requirements of the chrysanthemum leafminer,Chromatomyla syngenesiae (Diptera: Agromyzidae) and its ectoparasitoid,Diglyphus isaea (Hymenoptera: Eulophidae).Entomophaga 32:357–365.
Danehower, D. A., andBordner, J. 1984. Cuticular wax ofEpilachna varivestis.Insect Biochem. 14:671–676.
de Renobales, M., Nelson, D. R., andBlomquist, G. J. 1991. Cuticular lipids, pp. 240–251,in K. Binnington and A. Retnakaran (eds). Physiology of the Insect Epidermis. CSIRO, Australia.
Ferveur, J. F., Storkuhl, K. F., Stocker, R. F., Greespan, R. J. 1995. Genetic feminisation of brain structures and changed sexual orientation in maleDrosophila.Science 267:902–905.
Finidori-Logli, V. 1994. Ecologie chimique deDiglyphus isaea (Hymenoptera: Eulophidae).Thèse de l'Université de Provence. Marseille, France. 124 pp.
Finidori-Logli, V., Bagnères, A. G., Clément, J. L. 1996. Role of plant volatiles in the search for a host by parasitoidDiglyphus isaea (Hymenoptera: Eulophidae).J. Chem. Ecol. 22:541–558.
Francke, W. 1986. Convergency and diversity in multicomponent insect pheromones, pp. 327–336,in M. Porchet, J. C. Andries, and A. Dhainaud (eds.). Advances in Invertebrate Reproduction 4. Elsevier Science, Amsterdam.
Francke, W., Schroder, W., Bergström, G., Tengö, J. 1984. Esters in the volatile secretion of bees.Nova Acta Regiae Soc. Sci. Ups. Ser. V C 3:127–136.
Hadley, N. F. 1981. Cuticular lipids of terrestrial plants and arthropods: A comparison of their structure, composition and waterproofing barrier.J. Exp. Zool. 222:239–248.
Hegdekar, B. M., andDondale, C. D. 1969. A contact sex pheromone and some response parameters in lycosid spiders.Can. J. Zool. 47:1–4.
Howard, R. W. 1992. Comparative analysis of cuticular hydrocarbons from the ectoparasitoidsCephalomia waterstoni andLaelius utilis (Hymenoptera: Bethylidae) and their respective hosts,Cryptolestes ferrugineus (Coleoptera: Cucujidae) andTrogoderma variabile (Coleoptera: Dermestidae).Ann. Entomol. Soc. Am. 85:317–325.
Howard, R. W. 1993. Cuticular hydrocarbons and chemical communication, pp. 179–226,in D. W. Stanley-Samuelson and D. R. Nelson (eds.). Insect Lipids: Chemistry, Biochemistry and Biology. University of Nebraska Press, Lincoln.
Howard, R. W., andLiang, Y. 1993. Cuticular hydrocarbons of winged and wingless morphs of the ectoparasitoidChoetospila elegans Westwood (Hymenoptera: Pteromalidae) and its host, larval lesser grain borerRhyzopertha dominica (Coleoptera: Bostrichidae).Comp. Biochem. Physiol. 106B:407–414.
Howard, R. W., McDaniel, C. A., Nelson, D. R., Blomquist, G. J., Gelbaum, L. T., andZalkow, L. H. 1982. Cuticular hydrocarbons ofReticulitermes viginicus (Banks) and their role as potential species and caste recognition cues.J. Chem. Ecol. 8:1227–1239.
Huyton, P. M., Langley, P. A., Carlson, D. A., andSchwarz, M. 1980. Specificity of contact sex pheromones in tsetse flies,Glossina spp.Physiol. Entomol. 5:253–264.
Jackson, L. L. 1981. Cuticular lipids of insects. IX. Surface lipids of the grasshoppers,Melanoplus bivittatus, Malanoplus femurrubrum, andMelanoplus dawsoni.Comp. Biochem. Physiol. 70B:441–445.
Kainoh, Y. 1986. Mating behavior ofAscogaster reticulatus Watanabe (Hymenoptera: Braconidae), an egg-larval parasitoid of the smaller tea tortrix moth,Adoxophyes sp. (Lepidoptera: Tortricidae). I. Diel patterns of emergence and mating and some conditions for mating.Appl. Entomol. Zool. 21:1–7.
Lockey, K. H. 1985. Insect cuticular lipids.Comp. Biochem. Physiol. 81B:263–273.
Lockey, K. H. 1988. Lipids of the insect cuticle: Origin, composition and function.Comp. Biochem. Physiol. 89B:595–645.
Lyon, J. P. 1976. Les populations aphidiennes en serre et leur limitation par utilisation expérimentale de divers entomophages.Acta Hortic. 58:405–409.
Matthews, R. W. 1974. Biology of Braconidae.Annu. Rev. Entomol. 19:15–32.
Minkenberg, O. P. J. M. 1989. Temperature effects on life history of the eulophid waspD. isaea, an ectoparasitoid of leafminers (Liriomyza spp.) on tomatoes.Ann. Appl. Biol. 115:381–397.
Miwa, T. K. 1963. Identification of peaks in gas-liquid chromatography.J. Am. Oil. Chem. Soc. 40:309–313.
Schal, C., Gu, X., Burns, E. L., Blomquist, G. J. 1994. Pattern of biosynthesis and accumulation of hydrocarbons and contact sex pheromone in the female German cockroach,Blatella germanica.Arch. Insect Biochem. Physiol. 25:375–391.
Schmitt, U., Lubke, G., andFrancke, W. 1991. Tarsal secretion marks food sources in bumble bees (Hymenoptera: Apidae).Chemoecology 2:35–40.
Sick, M., Ayasse, M., Tengö, J., Engels, W., Lubke, G., andFrancke, W. 1994. Host-parasite relationships in six species ofSphecodes bees and their Halictid host: nest intrusion, intranidal behavior, and Dufour's gland volatiles (Hymenoptera: Halictidae).J. Chem. Ecol. 7:101–117.
Suzuki, Y., andHiehatri, K. 1985. Mating systems and sex ratios in the egg parasitoids,Trichogramma dendrolini andT. papilionis (Hymenoptera: Trichogrammatidae).Anim. Behav. 33:1223–1227.
Takahashi, S. Hajika, M., Takabayashi, J., andFukui, M., 1990. Oviposition stimulants in the coccoid cuticular waxes ofAphitis yanonensis De Bach & Rosen,J. Chem. Ecol. 16:1657–1667.
Tengo, J., Groth, I., andBergström, G. 1985. Volatile secretions in three species of Dufourea (Hymenoptera: Halictidae) bees: Chemical composition and phylogeny.Z. Naturforsch. 40c:657–660.
Tengö, J., Hefetz, A., Bertsch, A., Schmitt, U., Lubke, G., andFrancke, W. 1991. Species specificity and complexity of Dufour's gland secretion of bumble bees.Comp. Biochem. Physiol. 99B:641–646.
Uebel, E. C., Sonnet, P. E., Menzer, R. E., Miller, R. W., andLusby, W. R. 1977. Mating-stimulant pheromone and cuticular lipid constituents of the little house fly,Fannia canicularis (L.).J. Chem. Ecol. 3:269–278.
Warthen, J. D., andUebel, E. C. 1980. Differences in the amounts of two major cuticular esters in males, females and nymphs ofMelanoplus differentialis Thomas (Orthoptera: Acrididae).Acrida 9:101–106.
Warthen, J. D., Uebel, E. C., Lusby, W. R., andAdler, V. E. 1981. The cuticular lipids of the walkingstick,Diapheromera femorata Say.Insect Biochem. 11:467–472.
Weygand, A., andHilgetag, G. 1970. Organisch-chemische Experimentierkunst 4, Aufl.,in G. Hilgetag and A. Martin (eds.). Joh. Ambrosius Barth, Leipzig, 1216 p.
Wicker, C., andJallon, J. M. 1995a. Hormonal control of sex pheromone biosynthesis inDrosophila melanogaster.J. Insect Physiol. 4:65–70.
Wicker, C., andJallon, J. M. 1995b. Influence of ovary and ecdysteroids on pheromone biosynthesis inDrosophila melanogaster.Eur. J. Entomol. 92:197–202.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Finidori-Logli, V., Bagnères, AG., Erdmann, D. et al. Sex recognition inDiglyphus isaea walker (Hymenoptera: Eulophidae): Role of an uncommon family of behaviorally active compounds. J Chem Ecol 22, 2063–2079 (1996). https://doi.org/10.1007/BF02040095
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02040095