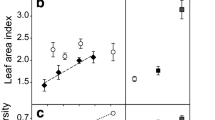
Abstract
A combination of optical measurements of leaf heights and observations on litterfall provided a vertical and temporal description of the leaf community structure in a tall, Liriodendron forest on the Maryland coastal plain. Leaf area, mass, and number were bimodally distributed with height. Median leaf number occurs far below (7–8 m) and median leaf mass far above (22–23 m) the median leaf area (18–19 m). Tree species exhibited leaf stratification into 3 height levels: understory (0–10 m), mid canopy (10–25 m), and overstory (25–37 m). Species leaf area in litterfall was related to the species basal area, although representation of leaf number in litterfall was not correlated with stem numbers for species in the stand. Species also showed a clear phenological sequence of leaf fall.
Similar content being viewed by others
Abbreviations
- LAD =:
-
Leaf Area Duration
- LAI =:
-
Leaf Area Index
- SLA =:
-
Specific Leaf Area
References
Aber J. D. 1979. A method for estimating foliage-height profiles in broad-leaved forests. J. Ecol. 67: 35–40.
Anderson M. C. 1966. Stand structure and light penetration. II. A theoretical analysis. J. Appl. Ecol. 3: 41–51.
Bongers F., Popma J., delCastillo J. Meave & Carabias J. 1988. Structure and floristic composition of the lowland rain forest of Los Tuxtlas, Mexico. Vegetatio 74: 55–80.
Bray J. R. 1964. Primary consumption in three forest canopies. Ecology 45: 165–167.
Bray J. R. & Gorham E. 1964. Litter production in forests of the world. Adv. Ecol. Res. 2: 101–157.
Brush G. S., Lenk C. & Smith J. 1980. The natural forests of Maryland: an explanation of the vegetation map of Maryland. Ecol. Monogr. 50: 77–92.
Campbell G. S. 1977. An introduction to environmental biophysics. Springer-Verlag, New York.
Carlisle A., Brown A. H. F. & White E. J. 1966. Litterfall, leaf production and the effects of defoliation by Tortrix viridana in a sessile oak (Quercus petraea) woodland. J. Ecol. 54: 65–85.
Carroll G. L. 1980. Forest canopies: complex and independent subsystems. In: Waring R. H. (ed.), Forests: fresh perspectives from ecosystem analysis, pp. 87–107. Oregon State University Press, Corvallis, OR.
Chirlin G. R. & Schaffner R. W. 1977. Observations on the water balance for seven sub-basins of the Rhode River, Maryland. In: Correll D. L. (ed.), Watershed research in eastern North America, pp. 277–306. Smithsonian Press, Washington, D.C.
Correll D. L. 1977. An overview of the Rhode River Watershed Program. In: Correll D. L. (ed.), Watershed research in eastern North America, pp. 105–124. Smithsonian Press, Washington, D.C.
Dixon K. R. 1976. Analysis of seasonal leaf fall in north temperate deciduous forests. Oikos 27: 300–306.
Eyre F. H. (ed.) 1980. Forest cover types of the United States and Canada. Society of American Foresters, Washington, D.C.
Ferguson D. K. 1985. The origin of leaf assemblages-new light on an old problem. Rev. Palaeobot. Palynol. 46: 117–188.
Hubbell S. P. & Foster R. B. 1986. Canopy gaps and the dynamics of a neotropical forest. In: Crawley M. J. (ed.), Plant ecology, pp. 77–96. Blackwell Scientific Publishers, Boston.
Hughes M. K. 1971. Tree biocontent, reproduction and litter fall in a deciduous woodland. Oikos 22: 62–73.
Ishizuka M. 1984. Spatial patterns of trees and their crowns in natural mixed forests. Jap. J. Ecol. 34: 421–430.
Jackson J. A. 1979. Tree surfaces as foraging substrates for insectivorous birds. In: Dickson J. G. et al. (eds.), The role of insectivorous birds in forest ecosystems, pp. 69–93, Academic Press, New York.
Jackson L. W. R. 1967. Effects of shade on leaf structure of deciduous tree species. Ecology 48: 498–499.
Larcher W. 1980. Physiological plant ecology (translated by Biederman-Thorson, M. A.). Springer-Verlag, Berlin.
Lechowicz M. J. 1984. Why do temperate deciduous forest trees leaf out at different times? Adaptation and ecology of forest communities. Am. Nat. 124: 821–842.
Lee R. 1978. Forest microclimatology. Columbia University Press, N.Y.
Lindroth A. & Halldin S. 1986. Numerical analysis of pine forest evaporation and surface resistance. Agric. For. Met. 38: 59–79.
Lull M. W. 1968. A forest atlas of the northeast. USDA Forest Service. Northeastern Forest Experiment Station, Upper Darby, PA.
MacArthur R. H. & Horn H. S. 1969. Foliage profile by vertical measurements. Ecology 50: 802–804.
Miller P. C. 1967. Tests of solar radiation models in three forest canopies. Ecology 50: 878–885.
Pierce J. W. 1982. Geology and soils of the Rhode River Watershed. In: Correll D. L. (ed.), Environmental data summary for the Rhode River Ecosystem. Vol. A, Part I. pp. 181–216. Smithsonian Environmental Research Center, Edgewater, MD.
Popma J., Bongers F. & delCastillo J. Meave. 1988. Pattern in the vertical structure of the tropical lowland rainforest of Los Tuxtlas, Mexico. Vegetatio 74: 81–91.
Reichle D. E., Goldstein R. A., VanHookJr. R. I. & Dodson G. J. 1973. Analysis of insect consumption in a forest canopy. Ecology 54: 1076–1084.
SAS Institute, Inc. 1985. SAS Users Guide: Statistics, Version 5 Edition. SAS Institute Inc., Cary, NC.
Schowalter T. D., Hargrove W. W. & CrossleyJr. D. A. 1986. Herbivory in forested ecosystems. Ann. Rev. Entomol. 31: 177–196.
Schulze E. D., Fuchs M. I. & Fuchs M. 1977. Spacial distribution of photosynthetic capacity and performance in a mountain spruce forest of northern Germany. I. Biomass distribution and daily CO2 uptake in different crown layers. Oecologia 29: 43–67.
Shinozaki K., Yoda K., Hozumi K. & Kira T. 1964a. A quantitative analysis of plant form-the pipe model theory, I. Basic analyses. Jap. J. Ecol. 14: 97–105.
Shinozaki K., Yoda K., Hozumi K. & Kira T. 1964b. A quantitative analysis of plant form-the pipe model theory, II. Further evidence of the theory and its application in forest ecology. Jap. J. Ecol. 14: 133–139.
Smith A. P. 1973. Stratification of temperate and tropical forests. Am. Nat. 107: 671–683.
Strong D. R. 1977. Epiphyte loads, treefalls, and perennial forest disruption: a mechanism for maintaining higher tree species richness in the tropics without animals. J. Biogeogr. 4: 215–218.
Waring R. M. 1983. Estimating forest growth and efficiency in relation to canopy leaf area. Adv. Ecol. Res. 13: 327–354.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Parker, G.G., O'Neill, J.P. & Higman, D. Vertical profile and canopy organization in a mixed deciduous forest. Vegetatio 85, 1–11 (1989). https://doi.org/10.1007/BF00042250
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00042250