Abstract
Background
The prognostic value of four proposed modifications to the 8th American Joint Committee on Cancer (AJCC) TNM staging system has yet to be evaluated. This study aimed to validate five proposed modifications.
Methods
Patients who underwent pancreatic ductal adenocarcinoma resection (2014–2016), as registered in the prospective Dutch Pancreatic Cancer Audit, were included. Stratification and prognostication of TNM staging systems were assessed using Kaplan–Meier curves, Cox proportional hazard analyses, and C-indices. A new modification was composed based on overall survival (OS).
Results
Overall, 750 patients with a median OS of 18 months (interquartile range 10–32) were included. The 8th edition had an increased discriminative ability compared with the 7th edition {C-index 0.59 (95% confidence interval [CI] 0.56–0.61) vs. 0.56 (95% CI 0.54–0.58)}. Although the 8th edition showed a stepwise decrease in OS with increasing stage, no differences could be demonstrated between all substages; stage IIA vs. IB (hazard ratio [HR] 1.30, 95% CI 0.80–2.09; p = 0.29) and stage IIB vs. IIA (HR 1.17, 95% CI 0.75–1.83; p = 0.48). The four modifications showed comparable prognostic accuracy (C-index 0.59–0.60); however, OS did not differ between all modified TNM stages (ns). The new modification, migrating T3N1 patients to stage III, showed a C-index of 0.59, but did detect significant survival differences between all TNM stages (p < 0.05).
Conclusions
The 8th TNM staging system still lacks prognostic value for some categories of patients, which was not clearly improved by four previously proposed modifications. The modification suggested in this study allows for better prognostication in patients with all stages of disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Although recent advancements in pancreatic cancer treatment have led to more effective systemic therapy, pancreatic ductal adenocarcinoma (PDAC) remains associated with a 5-year survival of about 10%.1 For patients with resectable, non-metastasized disease, pancreatic resection combined with (neo)adjuvant systemic therapy is considered the most optimal treatment strategy.2,3 However, even after resection and (neo)adjuvant chemotherapy, oncological outcomes remain unsatisfactory, with a median overall survival (OS) of only 22 months.3
The prognosis of PDAC strongly depends on various pathological factors of the surgical specimen, including tumor size and metastatic lymph nodes, as well as any distant metastases.4,5,6,7,8,9 Consequently, staging after surgery is important for accurate survival predictions, to guide the direction of treatment strategies and to inform patients on their prognosis.10,11 To describe the extent of disease progression in patients with different types of cancer, the American Joint Committee on Cancer (AJCC) TNM staging system is commonly used.12,13 In 2018, the 8th edition of the AJCC TNM classification for PDAC was introduced, which resulted in adjustments regarding primary tumor (T) and regional lymph node (N) stage. The T3 stage, previously defined as ‘tumor extension beyond the pancreas’, was changed to ‘tumors > 4 cm’, as the former definition could be interpreted differently by pathologists and lacked prognostic correlation.12,13,14 The extent of nodal involvement was subdivided from N1 ‘regional lymph node metastasis’ into N1 ‘metastases in 1–3 regional lymph nodes’, and N2 ‘metastases in ≥ 4 regional lymph nodes’.12,13
The 8th edition of the AJCC TNM classification has been previously validated in high-volume pancreatic centers15,16,17,18,19,20,21,22,23,24,25,26; however, the general applicability of the revised classification, in particular to low-volume centers, remains unclear. To this purpose, validation in a nationwide setting is desired. In addition, four recent studies have proposed further modifications of the 8th TNM classification, suggesting increased discriminative power.20,21,22,23,24,25,26 Furthermore, a subdivision of N stage based on metastatic lymph node ratio (LNR), accounting for the total number of examined lymph nodes, was suggested for a more accurate prediction of survival.24,27
This study aims to evaluate and further improve the prognostic value and general applicability of the 8th TNM staging system for PDAC and proposed modifications in a nationwide, unselected cohort of patients.
Methods
Study Design
A nationwide, multicenter observational cohort study was conducted in all 16 centers for pancreatic cancer surgery in The Netherlands. Included were all patients who underwent resection of histologically proven PDAC between 2014 and 2016, as registered in the nationwide, mandatory, prospective Dutch Pancreatic Cancer Audit (DPCA).28,29 Exclusion criteria were macroscopic irradical resection (R2 resection) and death within 90 days after resection. Furthermore, patients who received neoadjuvant treatment were excluded, considering that consensus on optimal pathology assessment after neoadjuvant chemo(radio)therapy is lacking and may thus influence TNM staging.30 Patients with T4 tumors were also excluded, as stage T4 indicates unresectable tumors according to the most recent AJCC definition due to arterial tumor involvement.13,20 Moreover, the majority of these patients were considered to have locally advanced disease and were therefore initially treated with neoadjuvant chemotherapy.31
Data Collection
After approval from the Dutch Pancreatic Cancer Group Scientific Committee, prospective baseline and perioperative data were retrieved from the clinical audit.32 The Charlson Age-Comorbidity Index (CACI) was calculated using the MDCalc CCI calculator.33 No data on race or ethnicity of study participants were obtained, as these data are not available in the DPCA. Survival data were retrieved from the patients’ hospital record. In addition, histopathological reports were obtained to collect detailed pathology data retrospectively. This information was used to assess T, N, and TNM stages according to the 7th and 8th AJCC definitions, based on tumor size, tumor extension, and lymph node involvement.12,13 Tumor size comprised the maximal tumor diameter in centimeters as mentioned in the pathology evaluation report, preferably measured microscopically. Resections were considered margin-positive if tumor cells were present within 1 mm of each microscopically assessed margin, apart from the anterior margin.34 The LNR was calculated by dividing the number of positive lymph nodes by the total number of lymph nodes examined. In case of uncertainty, an expert pancreatic cancer pathologist was consulted.
Pathology data were furthermore used to validate modifications of the 8th AJCC edition, as recently proposed in the literature.23,24,25,26 These modifications changed the TNM stages by either combining parameters from the 7th and 8th AJCC staging systems, altering their respective substages, or adjusting the N stage based on LNR (Table 1). In addition, a new modification was composed from optimal regrouping of the TNM substages based on median OS in our cohort, maintaining T and N stage definitions according to the 8th AJCC edition.13
Statistical Analysis
Missing data were considered missing at random and handled by multiple imputation based on a Markov chain Monte Carlo method (5 imputations, 10 iterations).35 Categorical variables were presented as frequencies and percentages and were compared using the Chi-square test. Parametric continuous variables were presented as mean ± standard deviation (SD) and non-parametric continuous variables were presented as median (interquartile range [IQR]).
The primary outcome was OS, defined as the time from the date of primary tumor resection until the date of death from any cause. If survival data were missing, patients were censored from the date of their last follow-up. Survival rates after 1, 2, and 3 years were determined by patients with a known vital status at that respective time. Unadjusted OS was compared between and within the different T, N, and TNM stages of (proposed) staging systems using the Kaplan–Meier method and log-rank test, and presented as median with 95% confidence intervals (CIs). To assess the independent discriminative ability of the 8th T stage, Kaplan–Meier analysis was performed in node-negative patients only. Cox proportional hazard analyses were performed to obtain hazard ratios (HRs) with 95% CIs. To evaluate the discriminative power of the 7th and 8th TNM staging systems and their proposed modifications, concordance indices (C-index) were calculated. Statistical analyses were performed using R version 3.5.1 (Bell Laboratories, NH, USA), including the ‘survival’, ‘ggplot’ and ‘mice’ packages. A p value <0.05 was considered statistically significant.
Results
Study Population
A total of 750 patients who underwent PDAC resection were included, with a median follow-up time of 37 months (IQR 31–48 months) (Table 2). Mean age was 67 years (SD ± 9) and 402 patients (54%) were male. Mean pathological tumor size was 3.2 cm (SD ± 1.2). Median number of positive lymph nodes was 2 (IQR 0–5) and median number of examined lymph nodes was 15 (IQR 10–21). In 361 patients (48%), fewer than 15 lymph nodes were examined. Median OS of the entire cohort was 18 months (IQR 10–32 months), with a survival rate of 70, 41, and 29% after 1, 2, and 3 years of follow-up, respectively.
Distribution of Patients
Regrouping of PDAC patients according to the 8th TNM classification, compared with the 7th edition, was visualized using a Sankey diagram (Fig. 1). The distribution of patients among the subgroups of the 7th TNM classification was considerably skewed. Restaging according to the 8th edition resulted in a reclassification of 394 patients (53%), of whom 137 patients (35%) migrated to a lower stage and 257 patients (65%) migrated to a higher stage. The revision mainly affected 7th TNM stage IIB; of 548 patients, 291 patients (53%) remained in stage IIB according to the 8th AJCC edition, while 257 patients (47%) were reclassified as stage III. This was mainly due to differentiation of N stage in the 8th edition. Consequently, the 8th TNM classification showed a more even distribution.
Survival by T, N, and TNM Stages
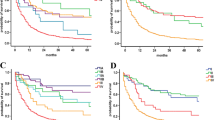
Both T and N stage of the 8th AJCC edition were discriminative for survival (Fig. 2a, b). A sensitivity analysis was performed, only using patients with at least 15 examined lymph nodes, which showed similar discrimination in survival for N stage (Fig. 2c). Kaplan–Meier and Cox proportional hazard analyses showed that a stepwise increase in TNM stage according to the 7th AJCC edition did not correspond with stepwise decrease in median OS (Fig. 3a). Stage IIA tumors were associated with a better OS than stage IB tumors, although this was not statistically significant (HR 0.63, 95% CI 0.36–1.10; p = 0.10). The 8th AJCC edition did show a sequential decline in OS from stage IA to III. However, no differences were found between stages IIA and IB (HR 1.30, 95% CI 0.80–2.09; p = 0.29) and stages IIB and IIA (HR 1.17, 95% CI 0.75–1.83; p = 0.48) (Fig. 3b).
Kaplan–Meier curves comparing overall survival between each a T stage of the 8th AJCC edition in 202 patients with node-negative disease; b N stage of the 8th AJCC edition in all 750 patients; and c N stage of the 8th AJCC edition in 389 patients with at least 15 examined lymph nodes. AJCC American Joint Committee on Cancer
Kaplan–Meier curves and results of Cox proportional hazard analyses comparing overall survival between patients classified according to the a 7th AJCC TNM classification; b 8th AJCC TNM classification; c modification of Jiang et al. 23; d modification of Li et al. 24; e modification of Shi et al. 25; and f modification of Pu et al. 26 HRs are calculated between each consecutive stage. AJCC American Joint Committee on Cancer, HR hazard ratio, CI confidence interval
Proposed Modifications of the 8th Edition
Overall, the modifications of Jiang et al.23, Li et al.24 and Pu et al.26 did not clearly improve the distribution of patients among the subgroups compared with the 8th AJCC edition within this nationwide cohort (electronic supplementary material [ESM] Appendices IA, B, and D); however, the modification of Shi et al.25 did show a slight improvement (ESM Appendix IC).
Comparable with the 8th edition, all four proposed modifications showed an overall stepwise decrease in OS with increasing stage (p < 0.001). However, according to the modification of Jiang et al.23 no statistically significant survival differences were found between stages IB and IA (HR 2.29, 95% CI 0.94–5.46; p = 0.07) and stages IIA and IB (HR 1.22, 95% CI 0.81–1.82; p = 0.34) (Fig. 3c). With the modification of Li et al.24 OS was not significantly different between stages IIA and IB (HR 1.30, (95% CI 0.80–2.09; p = 0.29) and between stages IIB and IIA (HR 1.23, 95% CI 0.80–1.91; p = 0.34) (Fig. 3d). Moreover, no survival differences were found between stages IIIA and IIB according to the modification of Shi et al.25 (HR 1.18, 95% CI 0.87–1.61; p = 0.27) (Fig. 3e). Using the modification of Pu et al.26 OS did not significantly differ for stages IIIB and IIIA (HR 1.20, 95% CI 0.88–1.63; p = 0.24) (Fig. 3f).
Prognostic Accuracy
The calculated C-indices for the 7th and 8th AJCC editions were 0.56 (95% CI 0.54–0.58) and 0.59 (95% CI 0.56–0.61), respectively (ESM Appendix II). From the proposed modifications, the modification by Shi et al.25 had the highest C-index of 0.60 (95% CI 0.57–0.62), compared with a C-index of 0.59 (95% CI 0.57–0.62) for Jiang et al.23, Li et al.24 and Pu et al.26
New Modification
Based on median OS within each TNM substage, optimal regrouping of patients was performed to compose a new modification, maintaining the 8th AJCC T and N definitions (Fig. 4, Table 1). Utilizing this modification, patients with T3N1 disease migrate from stage II to stage III. This classification resulted in a more even distribution of patients and showed a significant decrease in OS between all subsequent TNM stages (p < 0.015) (Fig. 5). The C-index was 0.59 (95% CI 0.57–0.62) (ESM Appendix II).
Discussion
This study validates the improved prognostic value and general applicability of the 8th AJCC TNM staging system after PDAC resection, compared with the 7th edition, in a nationwide, unselected cohort of patients. However, for patients with stage IIA disease, the prognostic value of the 8th TNM classification could still be questioned. In our cohort, the four proposed modifications of the 8th edition showed negligible improvement. Therefore, a new modification of the 8th AJCC edition was composed based on median OS of patients in our cohort, which resulted in optimal regrouping of the TNM substages.
The AJCC TNM staging system for malignant tumors is considered to be one of the most comprehensive tools for prognostication in patients with cancer in general.36,37 It allows investigators and doctors to communicate globally using a standardized language that reflects tumor burden. However, for PDAC patients, the TNM classification remains to be of moderate value.12,38 Accurate prediction of survival is important in cancer care as it helps to guide the direction of treatment decisions.10 Furthermore, the ability to correctly inform patients on their prognosis is crucial, in particular since the patients’ autonomy and shared decision making are increasingly being emphasized in current healthcare.11 Considering that recent advancements have resulted in more potent adjuvant treatment options for PDAC, such as FOLFIRINOX chemotherapy, accurate stratification of patients after PDAC resection might become of increasing importance.2
In recent years, the 8th AJCC TNM classification for PDAC was introduced, which showed major adjustments regarding the definitions of T and N stage.13 Using the former 7th edition, the majority of patients in our cohort was classified as stage IIA or IIB. This was mainly due to the large number of patients with T3 tumors, defined as tumors with ‘extension beyond the pancreas’.12 In contrast, with the 8th edition, a more even distribution among all TNM stages was found in our cohort, thus providing a better stratification for OS. Furthermore, the 8th AJCC classification showed a stage-dependent decrease in median OS, which was in contrast to its former edition.
However, with the 8th AJCC edition, OS remained indistinguishable between patients with stage IIA (including T3N0 patients) and stage IIB (including T1-3N1 patients) disease. This was in line with the findings of several other validation studies and may be explained by the relatively small number of T3N0 patients.15,22,25,26 Therefore, further improvement of the 8th TNM classification is mandatory. To this purpose, various modifications of the 8th edition were proposed, which showed only minor improvements within our cohort.19,23,24,25,26 Of these modifications, the regrouping scheme of Shi et al.25 using unchanged TNM parameters (Table 1) demonstrated the highest prognostic value.25 However, with their modification, OS could not be distinguished between patients with stage IIB and IIIA disease. Although our new modification, regrouping substages within stages II and III, showed a comparable discriminative power, significant OS differences between each revised subgroup were shown. Therefore, the newly proposed modification allows for better prognostication in patients with all stages of disease after PDAC resection.
Furthermore, various studies reported conflicting results with regard to the independent prognostic value of T and N stage. A dual-center study found that the revised T stage improves prognostication, while the revised N stage did not.39 On the contrary, a multicenter study by van Roessel et al. did not find a correlation between OS and the revised T stage in node-negative patients, whereas the revised N stage provided accurate discrimination of OS.19 Nevertheless, most studies proposing modifications of the 8th TNM classification maintained the 8th AJCC definitions for T and N stage.25,26,40,41 In our cohort, the 8th T and N stages were both found to be associated with OS, thereby significantly adding to the prognostic ability of the TNM staging system.
The present study has some limitations. First, due to lack of standardization, not all centers used the same histopathological examination and reporting protocol. Nevertheless, the nationwide pathology registry and network (PALGA) released a pathology protocol for synoptic reporting of pancreatic cancer in 2015.42 As all pathology laboratories in The Netherlands are affiliated with this network, standardization was stimulated. Second, patients who received neoadjuvant treatment were excluded. Neoadjuvant chemo(radio)therapy induces reactive changes to the pancreatic specimen such as fibrosis, which complicates the macroscopic and microscopic assessment and measurement of the tumor.30,43 Consequently, the current results are not applicable to patients who were treated with neoadjuvant therapy. Although international consensus was recently reached on assessment of the pancreatic specimen after neoadjuvant therapy, future research should validate the TNM classification in this setting.44 Third, to accurately assess the N stage, and thus the overall TNM stage, a sufficient number of harvested lymph nodes is mandatory.45,46 A higher number of harvested lymph nodes decreases the likelihood of underestimating the N stage. According to the International Study Group of Pancreatic Surgery (IGSPS), it is recommended to harvest at least 15 lymph nodes during PDAC resection.47 According to this criterion, the total number of examined lymph nodes was insufficient for a substantial part of patients (48%) in our cohort. It might therefore be possible that the number of positive lymph nodes in our study population, and subsequently the true N1 and N2 rates, are actually higher than reported, although a sensitivity analysis only using patients with sufficient harvested lymph nodes showed similar discrimination for N stage. This could be a consequence of the nationwide design of the study, which may have led to heterogeneity in the extent of lymph node harvesting. Despite that this could have influenced our results, it reflects the TNM assessment in a ‘real world’ daily clinical practice and therefore increases the general applicability of our findings.
Conclusion
This study provides evidence on the importance of joint consideration of T and N stage, and helps to further improve the TNM classification for PDAC. Although the prognostic value and general applicability of the 8th AJCC TNM staging system is improved compared with its former 7th edition, it still lacks prognostic value for some categories of patients. We propose a modification that moves patients with T3N1 disease from stage II to stage III. This revision allows for a better stepwise prognostication, although external validation is required to determine its true prognostic value.
Change history
07 July 2022
A Correction to this paper has been published: https://doi.org/10.1245/s10434-022-12182-z
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30.
Conroy T, Hammel P, Hebbar M, et al. Canadian cancer trials group and the unicancer-GI–PRODIGE Group. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395–406.
Latenstein AEJ, van der Geest LGM, Bonsing BA, et al. Dutch Pancreatic Cancer Group. Nationwide trends in incidence, treatment and survival of pancreatic ductal adenocarcinoma. Eur J Cancer. 2020;125:83–93.
Murakami Y, Uemura K, Sudo T, et al. Number of metastatic lymph nodes, but not lymph node ratio, is an independent prognostic factor after resection of pancreatic carcinoma. J Am Coll Surg. 2010;211(2):196–204.
Bilici A. Prognostic factors related with survival in patients with pancreatic adenocarcinoma. World J Gastroenterol. 2014;20(31):10802–12.
Strobel O, Hinz U, Gluth A, et al. Pancreatic adenocarcinoma: number of positive nodes allows to distinguish several N categories. Ann Surg. 2015;261(5):961–9.
Komo T, Murakami Y, Kondo N, et al. Prognostic impact of para-aortic lymph node micrometastasis in pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2016;23(6):2019–27.
Tol JA, Brosens LAA, Van Dieren S, et al. Impact of lymph node ratio on survival in patients with pancreatic and periampullary cancer. Br J Surg. 2015;102(3):237–45.
Basturk O, Saka B, Balci S, et al. Substaging of lymph node status in resected pancreatic ductal adenocarcinoma has strong prognostic correlations: proposal for a revised N classification for TNM staging. Ann Surg Oncol. 2015;22(3):1187–95.
Gospodarowicz MK, Miller D, Groome PA, et al. The process for continuous improvement of the TNM classification. Cancer. 2004;100(1):1–5.
Greene FL, Sobin LH. The TNM system: our language for cancer care. J Surg Oncol. 2002;80(3):119–20.
Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th edn. New York, NY: Springer-Verlag; 2009.
Kakar S, Pawlik TM, Allen PJ, et al. AJCC cancer staging manual. 8th edn. New York, NY: Springer-Verlag; 2017.
Saka B, Balci S, Basturk O, et al. Pancreatic ductal adenocarcinoma is spread to the peripancreatic soft tissue in the majority of resected cases, rendering the AJCC T-stage protocol (7th Edition) inapplicable and insignificant: a size-based staging system (pT1 ≤2, pT2: >2-≤4, pT3: >4 cm) is more valid and clinically relevant. Ann Surg Oncol. 2016;23(6):2010–8.
Allen PJ, Kuk D, Castillo CF, et al. Multi-institutional validation study of the American Joint Commission on Cancer (8th Edition) changes for T and N staging in patients with pancreatic adenocarcinoma. Ann Surg. 2017;265:185–91.
Kamarajah SK, Burns WR, Frankel TL, et al. Validation the American Joint Commission on Cancer (AJCC) 8th edition staging system for patients with pancreatic adenocarcinoma: a surveillance, epidemiology and end results (SEER) analysis. Ann Surg Oncol. 2017;24:2023–2020.
Kassardjian A, Stanzione N, Donahue TR, et al. Impact of changes in the American Joint Committee on Cancer staging manual, for pancreatic ductal adenocarcinoma. Pancreas. 2019;48(7):876–82.
Kwon W, He J, Higuchi R, et al. Multinational validation of the American Joint Committee on Cancer 8th edition pancreatic cancer staging system in a pancreas head cancer cohort. J Hepatobiliary Pancreat Sci. 2018;25(9):418–27.
van Roessel S, Kasumova GG, Verheij J, et al. International validation of the eighth edition of the American Joint Committee on Cancer (AJCC) TNM staging system in patients with resected pancreatic cancer. JAMA Surg. 2018;153(12):e183617.
Shin DW, Lee J, Kim J, et al. Validation of the American Joint Committee on Cancer 8th edition staging system for the pancreatic ductal adenocarcinoma. Eur J Surg Oncol. 2019;45(11):2159–65.
Song M, Yoon SB, Lee IS, et al. Evaluation of the prognostic value of the new AJCC 8th edition staging system for patients with pancreatic adenocarcinoma; a need to subclassify stage III? Eur J Cancer. 2018;104:62–9.
Liu L, Xu HX, He M, et al. A novel scoring system predicts postsurgical survival and adjuvant chemotherapeutic benefits in patients with pancreatic adenocarcinoma: implications for AJCC-TNM staging. Surg. 2018;163:1280–94.
Jiang Y, Su Y, Chen Y, Li Z. Refining the American Joint Committee on Cancer staging scheme for resectable pancreatic ductal adenocarcinoma using recursive partitioning analysis. J Canc. 2017;8(14):2765.
Li HJ, Chen YT, Yuan SQ. Proposal of a modified American Joint Committee on Cancer staging scheme for resectable pancreatic ductal adenocarcinoma with a lymph node ratio-based N classification: a retrospective cohort study. Medicine (Baltimore). 2018;97(34):e12904.
Shi S, Hua J, Liang C, et al. Proposed modification of the 8th edition of the AJCC staging system for pancreatic ductal adenocarcinoma. Ann Surg. 2019;269(5):944–50.
Pu N, Yin L, Habib JR, et al. Optimized modification of the eighth edition of AJCC TNM staging system for resected pancreatic ductal adenocarcinoma. Fut Onc. 2019;5(30):3457–65.
Pyo JS, Kim NY, Son BK, Chung KH. Prognostic implication of pN stage subdivision using metastatic lymph node ratio in resected pancreatic ductal adenocarcinoma. Int J Surg Pathol. 2020;28(3):245–51.
Dutch Pancreatic Cancer Audit (DPCA). Available at: http://www.dica.nl/dpca. Accessed 1 Oct 2020.
van Rijssen LB, Koerkamp BG, Zwart MJ, et al. Dutch Pancreatic Cancer Group. Nationwide prospective audit of pancreatic surgery: design, accuracy, and outcomes of the Dutch Pancreatic Cancer Audit. HPB (Oxford). 2017;19(10):919–926.
Chatterjee D, Katz MH, Foo WC, et al. Prognostic significance of new AJCC tumor stage in patients with pancreatic ductal adenocarcinoma treated with neoadjuvant therapy. Am J Surg Pathol. 2017;41(8):1097.
Walma MS, Brada LJ, Patuleia SIS, et al. Treatment strategies and clinical outcomes in consecutive patients with locally advanced pancreatic cancer: a multicenter prospective cohort. Eur J Surg Oncol. 2021;47:699–707.
Strijker M, Mackay TM, Bonsing BA, et al. Establishing and coordinating a nationwide multidisciplinary study group: lessons learned by the Dutch pancreatic cancer Group. Ann Surg. 2020;271(4):e102–4.
Walker G. Charlson comorbidity index (CCI) calculator. MdCALC. Available at: https://www.mdcalc.com/charlson-comorbidity-index-cci. Accessed 1 Oct 2020.
Federatie Medische Specialisten. Richtlijn Pancreascarcinoom 2019. Available at: https://richtlijnendatabase.nl/richtlijn/pancreascarcinoom/startpagina.html. Accessed 1 Oct 2020.
Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59(10):1087–91.
Yun JK, Lee GD, Kim HR, et al. Validation of the 8th edition of the TNM staging system in 3,950 patients with surgically resected non-small cell lung cancer. J Thorac Dis. 2019;11(7):2955.
In H, Solsky I, Palis B, et al. Validation of the 8th edition of the AJCC TNM staging system for gastric cancer using the national cancer database. Ann Surg Oncol. 2017;24(12):3683–91.
Bilimora KY, Bentrem DJ, Ko CY, et al. Validation of the 6th-edition AJCC pancreatic cancer staging system: report from the national cancer database. Cancer. 2007;110:738–44.
Schlitter A, Jesinghaus M, Jäger C, et al. pT but not pN stage of the 8th TNM classification significantly improves prognostication in pancreatic ductal adenocarcinoma. Eur J Cancer. 2017;84:121–9.
Asano D, Nara S, Kishi Y, et al. A single-institution validation study of lymph node staging by the AJCC 8th edition for patients with pancreatic head cancer: a proposal to subdivide the N2 category. Ann Surg Oncol. 2019;26(7):2112–20.
Ren H, Wu CR, Qiu GT, Zhang LP, Aimaiti S, Wang CF. Equipping the American Joint Committee on Cancer staging for resectable pancreatic ductal adenocarcinoma with tumor grade: a novel staging system. J Oncol. 2020;2020:9093729.
Stichting PALGA. Landelijke PALGA protocol: pancreas 2020. Available at: https://www.palga.nl/assets/uploads/Protocollen/Pancreas.pdf. Accessed 1 Oct 2020.
Soer E, Brosens LAA, van de Vijver M, et al. Dilemmas for the pathologist in the oncologic assessment of pancreatoduodenectomy specimens. Virchows Archiv. 2018;472(4):533–43.
Janssen BV, Tutucu F, van Roessel S, et al. Amsterdam International Consensus Meeting: tumor response scoring in the pathology assessment of resected pancreatic cancer after neoadjuvant therapy. Mod Pathol. 2021;34(1):4–12.
Huebner M, Kendrick M, Reid-Lombardo KM, et al. Number of lymph nodes evaluated: prognostic value in pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16(5):920–6.
Vuarnesson H, Lupinacci RM, Semoun O, et al. Number of examined lymph nodes and nodal status assessment in pancreaticoduodenectomy for pancreatic adenocarcinoma. Eur J Surg Oncol. 2013;39(10):116–1121.
Tol JA, Gouma DJ, Bassi C, et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery. 2014;156(3):591–600.
Funding
This research was not supported by any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Thijs J. Schouten and Lois A. Daamen authors share first authorship.
Hjalmar C. van Santvoort and I. Quintus Molenaar authors share senior authorship.
The original online version of this article was revised: Figure 4 and the last sentence in the Proposed Modifications of the 8th Edition section were corrected.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schouten, T.J., Daamen, L.A., Dorland, G. et al. Nationwide Validation of the 8th American Joint Committee on Cancer TNM Staging System and Five Proposed Modifications for Resected Pancreatic Cancer. Ann Surg Oncol 29, 5988–5999 (2022). https://doi.org/10.1245/s10434-022-11664-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-11664-4