Abstract
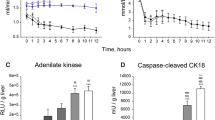
The hepatocellular metabolic change after liver transplantation following 2 hr cold ischemia was investigated. Of 55 orthotopic liver transplantations in male Wistar rats, 47 animals were sacrificed at 3 hr, and 1, 2, 7 and 30 days to determine hepatic metabolite levels, in the form of adenine nucleotides, lactate and glycogen. Using the other 8 recipients, biochemical examinations were done at 1, 3, 5, 7, 30 and 60 days and metabolic levels estimated at 60 days. The SGOT and SGPT levels decreased gradually after a remarkable increase on the first postoperative day, while the alkaline phosphatase level revealed a peak value at 30 days. All levels recovered to within the normal range in 60 days. The total adenine nucleotide level reached the normal range within 3 hr following the blood reflow and remained at a normal level thereafter. However, all the metabolic levels apart from total adenine nucleotides deteriorated to reach their worst level at 7 days. The results of this investigation indicate that the posttransplanted deterioration of metabolic levels were possibly caused by the imperfect oxygenation due to cellular edema after blood reflow. However, the levels of these metabolites recovered within 60 days after transplantation.
Similar content being viewed by others
References
Buhl MR. The postanoxic regeneration of 5′ adenine nucleotides in kidney tissue duringin vitro perfusion. Scand J Clin Lab Invest 1976; 36: 175–178.
Calman KC. The prediction of organ viability. 1 An hypothesis. Cryobiology 1974; 11: 1–6.
Isselhard W, Berger M, Denecke H, Witte J, Fischer JH, Molzberger H. Metabolism of canine kidneys in anaerobic ischemia and in aerobic ischemia by perfuffiation with gaseous oxygen. Pfluegers Arch 1972; 337: 87–106.
Collins GM, Taft P, Green RD, Ruprecht R, Halasz NA. Adenine nucleotide levels in preserved and ischemically injured canine kidneys. World J Surg 1977; 1: 237–243.
Fischer JH, Kulus D, Hansen-Schmidt I, Isselhard W. Adenine nucleotide levels of canine kidneys during hypothermic aerobic or anaerobic storage in Collins' solution. Eur Surg Res 1981; 13: 178–188.
Vogt MT, Farber E. On the molecular pathology of ischemic renal cell death. Am J Pathol 1968; 53: 1–26.
Warnick CT, Lazarus HM. The maintenance of adenine nucleotide levels during kidney storage in in tracellular solutions. Proc Soc Exp Biol Med 1979; 160: 453–457.
Pontegnie-Istace S, Lambotte L. Liver adenine nucleotide metabolism during hypothermic anoxia and a recovery period in perfusion. J Surg Res 1977; 23: 339–347.
Miyata M, Fischer JH, Fuhs M, Isselhard W, Kasai Y. A simple method for orthotopic liver transplantation in the rat. Transplantation 1980; 30: 335–338.
Lambotte L, Wojcik S. Measurement of cellular edema in anoxia and its prevention by hyperosmolar solutions. Surgery 1978; 83: 94–103.
Isselhard W, Merguet H. Metabolite des Glykolyse-Cyclus und des Adenylsaeure-Phosphokueatin-Systems im schlagenden und durch blutenden Warmblueterherzen unter verschiedenen Versuchsbedingungen. Pfluegers Arch ges Physiol 1962; 276: 211–235.
Isselhard W, Merguet H, Palm K. Bestimmung des Gesamtglykogens neben saeureloeslichen Metaboliten in Perchlorsaeure-Organhomoginaten. Z ges exp Med 1962; 136: 174–182.
Atkinson DE. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochem 1968; 7: 4030–4034.
Kamada N, Davies HS, Wight D, Culank L, Roser B. Liver transplantation in the rat. Buiochemical and histological evidence of complete tolerance induction in non-rejector strains. Transplantation 1983; 35: 304–311.
Howden B, Jablonski P, Grossman H, Marshall VC. The importance of the hepatic artery in rat liver transplantation. Transplantation 1989; 47: 428–431.
Coombes B, Schenker J. Laboratory tests. In Schiff L, Schiff E, editors: Diseases of the liver. Philadelphia: JB Lippincott, 1982.
Marni A, Ferrero ME, Gaja G. Metabolic function of grafted liver in rats. Transplantation 1988; 46: 830–835.
Newsholme EA, Gevers W. Control of glycolysis and gluconeogenesis in liver and kidney cortex. Vitam Horm 1967; 25: 1–87.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nakajima, Y., Kimura, J., Uchino, J. et al. Hepatocellular metabolic change after orthotopic liver transplantation in rats. The Japanese Journal of Surgery 21, 57–62 (1991). https://doi.org/10.1007/BF02470867
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02470867