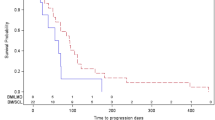
Abstract
Didemnin B 6.3 mg/m2 was administered intravenously to 48 patients with recurrent or progressive central nervous system tumors. One patient of 39 (2.9%, 95% confidence limits 0.1 to 13.5) eligible patients had a confirmed partial response utilizing standard solid tumor criteria which lasted 14 months. Toxicity was significant. Nausea and vomiting and lethargy were the most frequent toxicities, but multiple severe toxicities were seen. Further investigation of Didemnin B at this dose is not warranted in patients with central nervous system malignancies.
Similar content being viewed by others
References
Annual Report to the Food and Drug Administration on Didemnin B (NSC-3253219, IND-245055), August 1996
Dorr FA, Kuhn JG, Phillips J, Von Hoff DD: Phase I clinical and pharmacokinetic investigation of Didemnin B, a cyclic depsipeptide. J Cancer Clin Oncol 24: 1699–1706, 1988
Stewart JA, Low JB, Roberts JD, Blow A: A phase I clinical trial of Didemnin B. Cancer 68: 2550–2554, 1991
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Taylor, S.A., Giroux, D.J., Jaeckle, K.A. et al. Phase II study of Didemnin B in central nervous system tumors: A Southwest Oncology Group study. Invest New Drugs 16, 331–332 (1998). https://doi.org/10.1023/A:1006273214056
Issue Date:
DOI: https://doi.org/10.1023/A:1006273214056