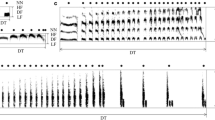
Abstract
A variety of bioacoustics distance metrics have been used to assess similarities in the vocalizations of different individuals. Here, we provide a detailed analysis of several acoustic similarity indices, some of which have been developed with the specific aim of characterizing the sensory coding of auditory stimuli. We compare different approaches through the analysis of begging calls of several passerine species and specialist brood parasitic cuckoos that putatively evolved to mimic their hosts. The different bioacoustics distances did not provide consistently correlated similarity patterns, implying that they are sensitive to different sound features. However, the encoded spectrogram alignment method was correlated with all other acoustic distance metrics, suggesting that this method provides a consistent approach to use when the perceptually salient sound parameters are unknown for a particular species. Our analyses confirm that statistical similarity of begging calls can be detected in a New Zealand pair of host and specialist parasite species. We also show detectable similarity in two other Australasian host–parasite pairs and another New Zealand system, but to a more limited extent. By examining phylogenetic patterns in the begging call diversity, we also confirm that specialist cuckoos have evolved to mimic the begging calls of their hosts but host species have not co-evolved to modify their calls in response to begging call similarity by the parasite. Our results illustrate that understanding the function and mechanism of behavioral copying and mimicry requires statistically consistent measures of similarity that are related to both the physical aspects of the particular display and the sensory basis of its perception.


Similar content being viewed by others
References
Anderson MG, Hauber ME (2007) A recognition-free mechanism for reliable rejection of brood parasites. Trends Ecol Evol 22(6):283–286
Anderson MG, Moskát C, Bán M, Grim T, Cassey P, Hauber ME (2009a) Egg eviction imposes a recoverable cost of virulence in chicks of a brood parasite. PLoS One 4(11):e7725
Anderson MG, Ross HA, Brunton DH, Hauber ME (2009b) Begging call matching between a specialist brood parasite and its host: a comparative approach to detect coevolution. Biol J Linn Soc 98:208–216
Baker MC, Logue DM (2003) Population differentiation in a complex bird sound: a comparison of three bioacoustical analysis procedures. Ethology 109:223–242
Briskie JV, Martin PR, Martin TE (1999) Nest predation and the evolution of nestling begging calls. Proc R Soc Lond B 266:2153–2159
Brooker MG, Brooker LC (1989) Cuckoo hosts in Australia. Aust Zool Rev 2:1–67
Brown JC, Smaragdis P (2009) Hidden Markov and Gaussian mixture models for automatic call classification. J Acoust Soc Am 125(6):EL221–EL224
Butchart SHM, Kilner RM, Fuisz T, Davies NB (2003) Differences in the nestling begging calls of hosts and host-races of the common cuckoo, Cuculus canorus. Anim Behav 65(2):345–354
Cardoso GC, Gama P, Depraz V (2007) Female and male serins (Serinus serinus) respond differently to derived song traits. Behav Ecol Sociobiol 61(9):1425–1436
Charif RA, Clark CW, Fristrup KM (2007) Raven Pro 1.3 User's manual. Cornell Laboratory of Ornithology, Ithaca, NY. edn
Christidis L, Boles WE (2008) Systematics and taxonomy of Australian birds. Collingwood, Vic. : CSIRO Pub.
Clemins PJ, Johnson MT (2006) Generalized perceptual linear prediction features for animal vocalization analysis. J Acoust Soc Am 120(1):527–534
Davies NB (2000) Cuckoos, cowbirds and other cheats. T & A D Poyser, London
Davies NB, Brooke De L M, Kacelnik A (1996) Recognition errors and probability of parasitism determine whether reed warblers should accept or reject mimetic eggs. Proc R Soc Lond B Biol Sci 263(1372):925–931
Davis SB, Mermelstein P (1980) Comparison of parametric representations for monosyllabic word recognition in continuously spoken sentences. IEEE transactions on acoustics, speech, and signal processing 28(4):357–365
Gill BJ (1983) Brood parasitism by the shining cuckoo Chrysococcyx lucidus at Kaikoura, New Zealand. Ibis 125:40–55
Gill P, Zhang J, Woolley SMN, Fremouw T, Theunissen FE (2006) Sound representation methods for spectro-temporal receptive field estimation. J Comput Neurosci 21(1):5–20
Grim T (2005) Mimicry vs. similarity: which resemblances between brood parasites and their hosts are mimetic and which are not? Biol J Linn Soc 84:69–78
Grim T (2006) The evolution of nestling discrimination by hosts of parasitic birds: why is rejection so rare? Evol Ecol Res 8:785–802
Grim T, Kleven O, Mikulica O (2003) Nestling discrimination without recognition: a possible defence mechanism for hosts towards cuckoo parasitism? Proc R Soc Lond B 270(suppl 1):S73–S75
Guerra JE, Cruz-Nieto J, Ortiz-Maciel SG, Wright TF (2008) Limited geographic variation in the vocalizations of the endangered thick-billed parrot: implications for conservation strategies. The Condor 110(4):639–647
Hauber ME (2003) Lower begging responsiveness of host versus parasitic brown-headed cowbird (Molothrus ater) nestlings is related to species identity but not to early social experience. J Comp Psychol 117(1):24–30
Hauber ME, Kilner RM (2007) Who mimics whom? communication, co-evolution, and chick mimicry in parasitic finches. Behav Ecol Sociobiol 61:497–503
Hauber ME, Cassey P, Woolley SMN, Theunissen FE (2007) Neurophysiological response selectivity for conspecific songs over synthetic sounds in the auditory forebrain of non-singing female songbirds. J Comp Physiol A Sens Neural Behav Physiol 193:765–774
Honza M, Vošlajerová K, Moskát C (2007) Eviction behaviour of the common cuckoo Cuculus canorus chicks. J Avian Biol 38(3):385–389
Hsu A, Woolley SMN, Fremouw TE, Theunissen FE (2004) Modulation power and phase spectrum of natural sounds enhance neural encoding performed by single auditory neurons. J Neurosci 24(41):9201–9211
Kogan JA, Margoliash D (1998) Automated recognition of bird song elements from continuous recordings using dynamic time warping and hidden markov models: a comparative study. J Acoust Soc Am 103(4):2185–2196
Langmore NE, Kilner RM (2007) Breeding site and host selection by Horsfield's bronze-cuckoos, Chalcites basalis. Anim Behav 74:995–1004
Langmore NE, Hunt S, Kilner RM (2003) Escalation of a coevolutionary arms race through host rejection of brood parasitic young. Nature 422(6928):157–160
Langmore NE, Maurer G, Adcock GJ, Kilner RM (2008) Socially acquired host-specific mimicry and the evolution of host races in Horsfield's bronze-cuckoo Chalcites basalis. Evolution 62(7):1689–1699
Langmore NE, Cockburn A, Russell AF, Kilner RM (2009) Flexible cuckoo chick-rejection rules in the superb fairy-wren. Behav Ecol 20(5):978–984
Latham J (1802) Supplementum indicis ornithologici, sive systematis ornithologiae. G. Leigh, J. & S, Sotheby, London
Lesica NA, Grothe B (2008) Efficient temporal processing of naturalistic sounds. PLoS ONE 3(2):e1655
Lewicki MS (2002) Efficient coding of natural sounds. Nat Neurosci 5:356–363
Manly BFJ (1997) Randomization, bootstrap and Monte Carlo methods in biology. Chapman and Hall, London
Margoliash D (1983) Acoustic parameters underlying the responses of song-specific neurons in the white-crowned sparrow. J Neurosci 3:1039–1057
Matheron Nocolas, Aubin T, Vielliard J, da Silva M-L, Sebe F, Boscolo D (2008) Singing in the rain forest: how a tropical bird song transfers information. PLoS ONE 3(2):e1580
McLean IG (1988) Breeding behaviour of the long-tailed cuckoo on little barrier island. Notornis 35:89–98
McLean IG, Waas JR (1987) Do cuckoo chicks mimic the begging calls of their hosts? Anim Behav 35(6):1896–1898
Moskát C, Hauber ME (2007) Conflict between egg recognition and egg rejection decisions in common cuckoo (Cuculus canorus) hosts. Anim Cogn 10(4):1435–9448
Naugler C, Ratcliffe L (1992) A field test of the sound environment hypothesis of conspecific song recognition in American tree sparrows (Spizella arborea). Behaviour 123(3–4):314–324
Payne RB (2005) The Cuckoos. Oxford University Press, Oxford
Payne RB, Payne LL (1998) Nestling eviction and vocal begging behaviors in the Australian glossy cuckoos Chrysococcyx basalis and C. lucidus. In: Rothstein SI, Robinson S (eds) Parasitic birds and their hosts: Studies in coevolution. Oxford University Press, New York, pp 152–170
Ranjard L, Ross HA (2008) Unsupervised bird song syllable classification using evolving neural networks. J Acoust Soc Am 123(6):4358–4368
Sewall KB (2009) Limited adult vocal learning maintains call dialects but permits pair-distinctive calls in red crossbills. Anim Behav 77(5):1303–1311
Singh NC, Theunissen FE (2003) Modulation spectra of natural sounds and ethological theories of auditory processing. J Acoust Soc Am 114(6 Pt 1):3394–3411
Sokal RR, Rohlf FJ (1995) Biometry. W.H. Freeman and Co., New York
Soler M (2009) Co-evolutionary arms race between brood parasites and their hosts at the nestling stage. J Avian Biol 40(3):237–240
Tao J, Johnson MT, Osiejuk TS (2008) Acoustic model adaptation for ortolan bunting (Emberiza hortulana L.) song-type classification. J Acoust Soc Am 123(3):1582–1590
Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP (2000) A procedure for an automated measurement of song similarity. Anim Behav 59(5):1167–1176
The Mathworks, Natick, MA (2008) Matlab 7.7.0.471 (R2008b). URL http://www.mathworks.com
Trawicki MB, Johnson MT, Osiejuk TS (2005) Automatic song-type classification and speaker identification of Norwegian ortolan bunting (Emberiza hortulana) vocalizations. In: 2005 IEEE Workshop on Machine Learning for Signal Processing, pp 277–282, URL http://ieeexplore.ieee.org/servlet/opac? punumber = 10270
Vallejo EE, Cody ML, Taylor CE (2007) Unsupervised acoustic classification of bird species using hierarchical self-organizing maps. In: Randall M, Abbass HA, Wiles J (eds) Progress in artificial life, third Australian conference, ACAL 2007 gold coast, Australia, December 4–6, Springer Berlin/Heidelberg, lecture notes in computer science, vol 4828/2007, pp 212–221
Vyas A, Harding C, Borg L, Bogdan D (2009) Acoustic characteristics, early experience, and endocrine status interact to modulate female zebra finches' behavioral responses to songs. Horm Behav 55:50–59
Woolley SMN, Rubel EW (2002) Vocal memory and learning in adult bengalese finches with regenerated hair cells. J Neurosci 22(17):7774–7787
Woolley SMN, Fremouw TE, Hsu A, Theunissen FE (2005) Tuning for spectro-temporal modulations as a mechanism for auditory discrimination of natural sounds. Nat Neurosci 8(10):1371–1379
Young SJ (1994) The HTK hidden markov model toolkit: design and philosophy. entropic cambridge research laboratory. Ltd 2:2–44
Acknowledgements
We thank Phillip Cassey, Brian Gill, Tomas Grim, Rebecca Kilner, Todd Landers, Naomi Langmore, Arnon Lotem, Luis Ortiz Catedral, Kevin Parker, and Allen Rodrigo for discussions and comments. Fieldwork was conducted with permission from the Auckland Regional Council, Department of Conservation, and the Massey University Animal Ethics Committee. We are grateful for field assistance by many helpers, too numerous to mention here (see http://www.massey.ac.nz/dhbrunto/ppl/MichaelAnderson.htm). Funding was provided from a Bright Futures Top Achiever Scholarship, Massey University (M.G.A.), the National Geographic Society and the Human Frontier Science Program (to M.E.H.), and two New Zealand Marsden Fund Grants (M.E.H.; D.H.B. and H.A.R.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Soler
Louis Ranjard, Michael G. Anderson contributed equally to the research
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Online Resource 1
Rank order of the similarity to the begging calls of the parasitic shining cuckoo in New Zealand and its grey warbler host, for each acoustic distance measure. The parasite and its host are highlighted and the species of the genus Chalcites are marked (†, dagger). The names of the shining cuckoo hosts are in brackets and abbreviated for g.w., grey warbler; w.t., western thornbill; and y.r.t., yellow-rumped thornbill. (DOC 50 kb)
Online Resource 2
Rank order of the similarity to the begging calls of the parasitic shining cuckoo in Western Australia and its western thornbill host, for each acoustic distance measure. The parasite and its host are highlighted and the species of the genus Chalcites are marked (†, dagger). The names of the shining cuckoo hosts are in brackets and abbreviated for g.w., grey warbler; w.t., western thornbill; and y.r.t., yellow-rumped thornbill. (DOC 52 kb)
Online Resource 3
Rank order of the similarity to the begging calls of the parasitic Horsfield's bronze-cuckoo in Western Australia and its western thornbill host, for each acoustic distance measure. The parasite and its host are highlighted and the species of the genus Chalcites are marked (†, dagger). The names of the shining cuckoo hosts are in brackets and abbreviated for g.w., grey warbler; w.t., western thornbill; and y.r.t., yellow-rumped thornbill. (DOC 51 kb)
Online Resource 4
Rank order of the similarity to the begging calls of the parasitic long-tailed cuckoo in New Zealand and its yellowhead, whitehead, and brown creeper hosts, for each acoustic distance measure. The parasite and its host are highlighted and the species of the genus Chalcites are marked (†, dagger). The names of the shining cuckoo hosts are in brackets and abbreviated for g.w., grey warbler; w.t., western thornbill; and y.r.t., yellow-rumped thornbill. (DOC 54 kb)
Rights and permissions
About this article
Cite this article
Ranjard, L., Anderson, M.G., Rayner, M.J. et al. Bioacoustic distances between the begging calls of brood parasites and their host species: a comparison of metrics and techniques. Behav Ecol Sociobiol 64, 1915–1926 (2010). https://doi.org/10.1007/s00265-010-1065-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-010-1065-2